Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2018-03-16 , DOI: 10.1016/j.apcata.2018.03.010 Xuan Zhang , Rui Zhang , Xianghai Meng , Haiyan Liu , Zhichang Liu , Hao Ma , Chunming Xu , Peter A.A. Klusener
|
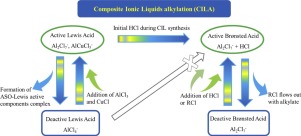
The deactivation mechanism and the activity recovery approach of composite ionic liquid (CIL), a kind of acidic chloroaluminate ionic liquids (ILs) modified with CuCl for the in 2013 successfully commercialized catalysis of isobutane alkylation are being discussed in this paper. CIL showed catalytic activity only in the presence of both Brønsted and Lewis acids. CIL deactivation was caused by the loss of Brønsted acidity and/or Lewis acidity. Brønsted acidity decreased by the loss of trace amount of formed chlorohydrocarbons with the outflowing alkylate. Lewis acidity was deactivated mainly due to the complexation of the formed acid soluble oil (ASO) with the CIL anions. Brønsted acidity was maintained by the continuous addition of hydrogen chloride (HCl) or tert-butyl chloride, and Lewis activity was recovered by AlCl3 addition. However, Brønsted acidity could not be recovered when Lewis acidity was lost and was thus dependent on Lewis acidity. Furthermore, ASO interacted with active components of CIL, causing the loss of copper via precipitation of CuCl which is the major contributor to the high selectivity. Therefore, CuCl was added simultaneously with AlCl3 to maintain the high selectivity of alkylate.
中文翻译:

复合离子液体异丁烷烷基化的失活机理和活性恢复方法
本文讨论了复合离子液体(CIL)的失活机理和活性恢复方法,该复合离子液体是一种CuCl改性的酸性氯铝酸盐离子液体(ILs),用于2013年成功商业化的异丁烷烷基化催化剂。CIL仅在布朗斯台德酸和路易斯酸同时存在时才显示催化活性。CIL失活是由布朗斯台德酸度和/或路易斯酸度的丧失引起的。布朗斯台德酸度由于流出的烷基化物损失了痕量形成的氯代烃而降低。路易斯酸的失活主要是由于形成的酸溶性油(ASO)与CIL阴离子的络合。通过连续添加氯化氢(HCl)或叔酸来保持布朗斯台德酸度-氯化丁酯,和路易斯活性通过添加AlCl 3恢复。但是,当路易斯酸丧失时,布朗斯台德酸度无法恢复,因此依赖于路易斯酸度。此外,ASO与CIL的活性成分相互作用,通过沉淀CuCl引起铜的损失,而CuCl是高选择性的主要贡献者。因此,与AlCl 3同时添加CuCl以保持烷基化物的高选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号