Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2018-03-16 , DOI: 10.1016/j.apcatb.2018.03.044 Minghui Zhu , Bin Li , Jih-Mirn Jehng , Lohit Sharma , Julian Taborda , Lihua Zhang , Eric Stach , Israel E. Wachs , Zili Wu , Jonas Baltrusaitis
|
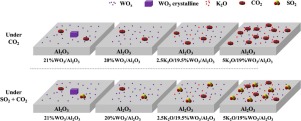
Molecular structures of the unpromoted and K2O-promoted supported WO3/Al2O3 catalysts were studied with in situ Raman and UV–vis spectroscopy. In situ Raman spectra revealed that supported 20% WO3/Al2O3 corresponds to near monolayer coverage of isolated and oligomeric surface WOx species on Al2O3. Above monolayer surface WOx coverage (21% WO3/Al2O3), crystalline WO3 nanoparticles are also present. The addition of K2O to the supported WO3/Al2O3 catalyst increased the concentration of isolated surface WOx species and did not form K2WO4 nanoparticles. The reducibility of the tungsten oxide structures depends on their structures (2D or 3D) and the K2O promoter. Their interaction with acidic CO2 and SO2 gases was also investigated. Adsorption of CO2 creates several surface carbonate species of varying acidity that were detected using a combination of in situ IR and mass spectroscopy. Adsorbed bicarbonate form on weakly basic surface sites on tungsten oxide monolayer WO3/Al2O3 catalyst as well as in the presence of low 2.5% K2O loading. At high 5% K2O loading, the presence of the strong surface basic sites yields adsorbed carbonates. After SO2 pretreatment, however, new strongly adsorbed sulfate appears on the surface that inhibits CO2 adsorption.
中文翻译:

负载型K 2 O / WO 3 / Al 2 O 3催化剂的分子结构和酸性气体表面化学
用原位拉曼光谱和紫外可见光谱研究了未助催化和K 2 O助催化的WO 3 / Al 2 O 3催化剂的分子结构。原位拉曼光谱显示支撑20%WO 3 / Al的2个ö 3对应于分离和低聚表面WO的附近单层覆盖X物种对Al 2 ö 3。单层表面上方的WO x覆盖率(21%WO 3 / Al 2 O 3),结晶WO 3纳米颗粒也存在。向负载的WO 3 / Al 2 O 3催化剂中添加K 2 O增加了分离的表面WO x物质的浓度,并且没有形成K 2 WO 4纳米颗粒。氧化钨结构的还原性取决于它们的结构(2D或3D)和K 2 O促进剂。还研究了它们与酸性CO 2和SO 2气体的相互作用。CO 2的吸附会产生几种酸度不同的表面碳酸盐物质,这些酸碳酸盐是通过原位结合检测出的红外和质谱。吸附的碳酸氢盐在氧化钨单层WO 3 / Al 2 O 3催化剂上的弱碱性表面部位以及低2.5%K 2 O负载下形成。在高5%K 2 O负载下,强表面碱性位点的存在会产生吸附的碳酸盐。然而,在SO 2预处理之后,新的强烈吸附的硫酸盐出现在抑制CO 2吸附的表面上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号