当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solution Nuclear Magnetic Resonance Studies of the Ligand-Binding Domain of an Orphan Nuclear Receptor Reveal a Dynamic Helix in the Ligand-Binding Pocket
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.biochem.8b00069 Nicolas Daffern 1 , Zhonglei Chen 1 , Yongbo Zhang 2 , Leslie Pick 3 , Ishwar Radhakrishnan 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.biochem.8b00069 Nicolas Daffern 1 , Zhonglei Chen 1 , Yongbo Zhang 2 , Leslie Pick 3 , Ishwar Radhakrishnan 1
Affiliation
|
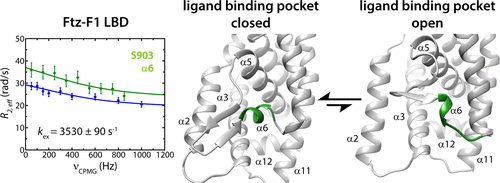
The ligand-binding domains (LBDs) of the NR5A subfamily of nuclear receptors activate transcription via ligand-dependent and ligand-independent mechanisms. The Drosophila Ftz-F1 receptor (NR5A3) belongs to the latter category, and its ligand independence is attributed to a short helical segment (α6) within the protein that resides in the canonical ligand-binding pocket (LBP) in the crystalline state. Here, we show that the α6 helix is dynamic in solution when Ftz-F1 is bound to the LxxLL motif of its cofactor Ftz, undergoing motions on the fast (picosecond to nanosecond) as well as slow (microsecond to millisecond) time scales. Motions on the slow time scale (∼10–3 s) appear to pervade throughout the domain, most prominently in the LBP and residues at or near the cofactor-binding site. We ascribe the fast time scale motions to a solvent-accessible conformation for the α6 helix akin to those described for its orthologs in higher organisms. We assign this conformation where the LBP is “open” to a lowly populated species, while the major conformer bears the properties of the crystal structure where the LBP is “closed”. We propose that these conformational transitions could allow binding to small molecule ligands and/or play a role in dissociation of the cofactor from the binding site. Indeed, we show that the Ftz-F1 LBD can bind phospholipids, not unlike its orthologs. Our studies provide the first detailed insights into intrinsic motions occurring on a variety of time scales in a nuclear receptor LBD and reveal that potentially functionally significant motions pervade throughout the domain in solution, despite evidence to the contrary implied by the crystal structure.
中文翻译:

孤核受体配体结合域的溶液核磁共振研究揭示了配体结合口袋中的动态螺旋
核受体的NR5A亚家族的配体结合域(LBD)通过配体依赖性和配体依赖性机制激活转录。在果蝇FTZ-F1受体(NR5A3)属于后一类,和其配体独立性归因于蛋白质中的短的螺旋段(α6)驻留在晶态的规范配体结合口袋(LBP)。在这里,我们显示当Ftz-F1绑定到其辅助因子Ftz的LxxLL基序时,α6螺旋在溶液中是动态的,在快速(皮秒至纳秒)和慢速(微秒至毫秒)的时间尺度上运动。慢速刻度上的运动(〜10 –3s)似乎遍及整个结构域,最显着的是在LBP和辅因子结合位点处或附近的残基。我们将快速时标运动归因于a6螺旋的溶剂可及构象,类似于在高等生物中直向同源物所描述的构象。我们将LBP“开放”的这种构象分配给人口少的物种,而主要构象体则具有LBP“封闭”的晶体结构的特性。我们提出这些构象转变可以允许与小分子配体结合和/或在辅因子从结合位点的解离中起作用。实际上,我们证明了Ftz-F1 LBD可以结合磷脂,与其直系同源物不同。
更新日期:2018-03-16
中文翻译:

孤核受体配体结合域的溶液核磁共振研究揭示了配体结合口袋中的动态螺旋
核受体的NR5A亚家族的配体结合域(LBD)通过配体依赖性和配体依赖性机制激活转录。在果蝇FTZ-F1受体(NR5A3)属于后一类,和其配体独立性归因于蛋白质中的短的螺旋段(α6)驻留在晶态的规范配体结合口袋(LBP)。在这里,我们显示当Ftz-F1绑定到其辅助因子Ftz的LxxLL基序时,α6螺旋在溶液中是动态的,在快速(皮秒至纳秒)和慢速(微秒至毫秒)的时间尺度上运动。慢速刻度上的运动(〜10 –3s)似乎遍及整个结构域,最显着的是在LBP和辅因子结合位点处或附近的残基。我们将快速时标运动归因于a6螺旋的溶剂可及构象,类似于在高等生物中直向同源物所描述的构象。我们将LBP“开放”的这种构象分配给人口少的物种,而主要构象体则具有LBP“封闭”的晶体结构的特性。我们提出这些构象转变可以允许与小分子配体结合和/或在辅因子从结合位点的解离中起作用。实际上,我们证明了Ftz-F1 LBD可以结合磷脂,与其直系同源物不同。











































 京公网安备 11010802027423号
京公网安备 11010802027423号