当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In Silico QT and APD Prolongation Assay for Early Screening of Drug-Induced Proarrhythmic Risk
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.jcim.7b00440 Lucia Romero 1 , Jordi Cano 1 , Julio Gomis-Tena 1 , Beatriz Trenor 1 , Ferran Sanz 2 , Manuel Pastor 2 , Javier Saiz 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.jcim.7b00440 Lucia Romero 1 , Jordi Cano 1 , Julio Gomis-Tena 1 , Beatriz Trenor 1 , Ferran Sanz 2 , Manuel Pastor 2 , Javier Saiz 1
Affiliation
|
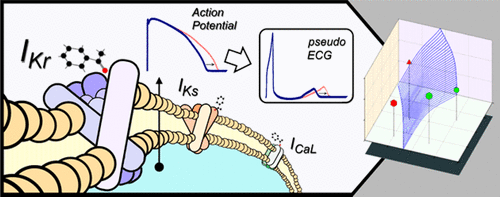
Drug-induced proarrhythmicity is a major concern for regulators and pharmaceutical companies. For novel drug candidates, the standard assessment involves the evaluation of the potassium hERG channels block and the in vivo prolongation of the QT interval. However, this method is known to be too restrictive and to stop the development of potentially valuable therapeutic drugs. The aim of this work is to create an in silico tool for early detection of drug-induced proarrhythmic risk. The system is based on simulations of how different compounds affect the action potential duration (APD) of isolated endocardial, midmyocardial, and epicardial cells as well as the QT prolongation in a virtual tissue. Multiple channel–drug interactions and state-of-the-art human ventricular action potential models (O’Hara, T., PLos Comput. Biol. 2011, 7, e1002061) were used in our simulations. Specifically, 206.766 cellular and 7072 tissue simulations were performed by blocking the slow and the fast components of the delayed rectifier current (IKs and IKr, respectively) and the L-type calcium current (ICaL) at different levels. The performance of our system was validated by classifying the proarrhythmic risk of 84 compounds, 40 of which present torsadogenic properties. On the basis of these results, we propose the use of a new index (Tx) for discriminating torsadogenic compounds, defined as the ratio of the drug concentrations producing 10% prolongation of the cellular endocardial, midmyocardial, and epicardial APDs and the QT interval, over the maximum effective free therapeutic plasma concentration (EFTPC). Our results show that the Tx index outperforms standard methods for early identification of torsadogenic compounds. Indeed, for the analyzed compounds, the Tx tests accuracy was in the range of 87–88% compared with a 73% accuracy of the hERG IC50 based test.
中文翻译:

用于药物诱发的心律失常风险的早期筛查的In Silico QT和APD延长检测
药物引起的心律失常是监管机构和制药公司的主要关注点。对于新药候选者,标准评估包括评估hERG钾通道阻滞和体内QT间隔延长。然而,已知该方法过于严格,并且阻止了潜在有价值的治疗药物的开发。这项工作的目的是创建一种计算机模拟工具,用于及早发现药物引起的心律失常风险。该系统基于不同化合物如何影响孤立的心内膜,心中膜和心外膜细胞的动作电位持续时间(APD)以及虚拟组织中QT延长的模拟。多通道药物相互作用和最新的人类心室动作电位模型(O'Hara,T.,PLos计算。生物学 2011, 7,e1002061)在我们的模拟中使用。具体而言,通过阻止延迟整流器电流(分别为I Ks和I Kr)和L型钙电流( I CaL)的慢和快分量来执行206.766细胞和7072组织模拟)处于不同的水平。通过对84种化合物的心律失常风险进行分类来验证我们系统的性能,其中40种具有致畸性。根据这些结果,我们建议使用一种新的指数(Tx)来区分致源性化合物,定义为引起心内膜,心中膜和心外膜APD延长10%的药物浓度与QT间隔的比值,超过最大有效游离治疗性血浆浓度(EFTPC)。我们的研究结果表明,Tx指数优于标准方法,可早日识别出致畸源性化合物。确实,对于所分析的化合物,与基于hERG IC 50的测试的73%的准确性相比,Tx测试的准确性在87-88%的范围内。
更新日期:2018-03-16
中文翻译:

用于药物诱发的心律失常风险的早期筛查的In Silico QT和APD延长检测
药物引起的心律失常是监管机构和制药公司的主要关注点。对于新药候选者,标准评估包括评估hERG钾通道阻滞和体内QT间隔延长。然而,已知该方法过于严格,并且阻止了潜在有价值的治疗药物的开发。这项工作的目的是创建一种计算机模拟工具,用于及早发现药物引起的心律失常风险。该系统基于不同化合物如何影响孤立的心内膜,心中膜和心外膜细胞的动作电位持续时间(APD)以及虚拟组织中QT延长的模拟。多通道药物相互作用和最新的人类心室动作电位模型(O'Hara,T.,PLos计算。生物学 2011, 7,e1002061)在我们的模拟中使用。具体而言,通过阻止延迟整流器电流(分别为I Ks和I Kr)和L型钙电流( I CaL)的慢和快分量来执行206.766细胞和7072组织模拟)处于不同的水平。通过对84种化合物的心律失常风险进行分类来验证我们系统的性能,其中40种具有致畸性。根据这些结果,我们建议使用一种新的指数(Tx)来区分致源性化合物,定义为引起心内膜,心中膜和心外膜APD延长10%的药物浓度与QT间隔的比值,超过最大有效游离治疗性血浆浓度(EFTPC)。我们的研究结果表明,Tx指数优于标准方法,可早日识别出致畸源性化合物。确实,对于所分析的化合物,与基于hERG IC 50的测试的73%的准确性相比,Tx测试的准确性在87-88%的范围内。









































 京公网安备 11010802027423号
京公网安备 11010802027423号