当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ni(COD)2-Catalyzed ipso-Silylation of 2-Methoxynaphthalene: A Density Functional Theory Study
Organometallics ( IF 2.5 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.organomet.8b00046 Pooja Jain 1 , Sourav Pal 1 , Vidya Avasare 2
Organometallics ( IF 2.5 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.organomet.8b00046 Pooja Jain 1 , Sourav Pal 1 , Vidya Avasare 2
Affiliation
|
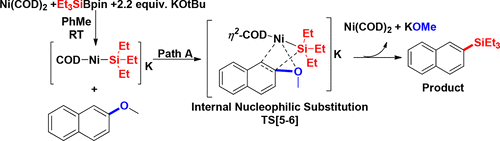
Density functional theory has been used for the systematic investigation of the mechanism involved in Ni(COD)2-catalyzed ipso-silylation of 2-methoxynaphthalene. The two fundamental mechanistic pathways, internal nucleophilic substitution and a nonclassical oxidative addition, have been studied. In both pathways, the first equivalent of KOtBu directly reacts with the silyl boronate (Et3SiBpin) to generate the silyl anion surrogate Et3SiK or silylborate [Et3Si-Bpin(OtBu)]K (IN3), which further reacts with Ni(COD)2 to form a substrate–catalyst complex, [(η2-COD)2NiSiEt3]K. The internal nucleophilic substitution reaction pathway proceeds through η2 complexation of nickel with the C(1)═C(2) bond of 2-methoxynaphthalene. Later, nickel connects to ═C(1) through σ-bond formation and coordinates with oxygen of the −OMe group. Simultaneously, the −SiEt3 group approaches ═C(2) possessing −OMe followed by rearomatization which is facilitated by coordination of K+ with nickel and methoxy oxygen. In a nonclassical oxidative addition, the chelation of K+ with −OMe as well as −SiEt3 from [(η2-COD)2NiSiEt3] is the key step which promotes the insertion of NiSiEt3 to the ═C(2) carbon of 2-methoxynaphthalene. We also observed that the activation energy barrier in the non-π-extended aromatic systems is higher than that of the π-extended aromatic systems. The overall study manifests that Ni(COD)2-catalyzed ipso-silylation of 2-methoxynapthalene operates through an internal nucleophilic substitution pathway.
中文翻译:

的Ni(COD)2催化的本位2-甲氧基萘的-Silylation的密度泛函理论研究
密度泛函理论已被用于从事镍(COD)机制的系统研究2催化的本位2-甲氧基的-silylation。已经研究了两个基本的机理途径,内部亲核取代和非经典氧化加成。在这两个途径中,KOtBu的第一等价物均与甲硅烷基硼酸酯(Et 3 SiBpin)直接反应,生成取代Et 3 SiK或甲硅烷基硼酸酯[Et 3 Si-Bpin(OtBu)] K(IN3)的甲硅烷基阴离子。的Ni(COD)2以形成基底催化剂络合物,[(η 2 -COD)2 NiSiEt 3] K。通过η内部的亲核取代反应途径进行2与C(1)= C(2)2-甲氧基萘的键络合的镍。后来,镍通过σ键形成与═C(1)连接,并与-OMe基团的氧配位。同时,-SiEt 3基团接近具有-OMe的═C(2),然后通过K +与镍和甲氧基氧的配位作用而被重新芳构化。在一个非经典氧化加成,K的螯合+与-OMe以及-SiEt 3从[(η 2 -COD)2 NiSiEt 3 ]是促进NiSiEt的插入的关键步骤3生成2-甲氧基萘的═C(2)碳。我们还观察到,非π扩展的芳族系统的活化能垒高于π扩展的芳族系统的活化能垒。整个研究该清单的Ni(COD)2催化的本位2-甲氧基萘的-silylation通过内部的亲核取代途径起作用。
更新日期:2018-03-17
中文翻译:

的Ni(COD)2催化的本位2-甲氧基萘的-Silylation的密度泛函理论研究
密度泛函理论已被用于从事镍(COD)机制的系统研究2催化的本位2-甲氧基的-silylation。已经研究了两个基本的机理途径,内部亲核取代和非经典氧化加成。在这两个途径中,KOtBu的第一等价物均与甲硅烷基硼酸酯(Et 3 SiBpin)直接反应,生成取代Et 3 SiK或甲硅烷基硼酸酯[Et 3 Si-Bpin(OtBu)] K(IN3)的甲硅烷基阴离子。的Ni(COD)2以形成基底催化剂络合物,[(η 2 -COD)2 NiSiEt 3] K。通过η内部的亲核取代反应途径进行2与C(1)= C(2)2-甲氧基萘的键络合的镍。后来,镍通过σ键形成与═C(1)连接,并与-OMe基团的氧配位。同时,-SiEt 3基团接近具有-OMe的═C(2),然后通过K +与镍和甲氧基氧的配位作用而被重新芳构化。在一个非经典氧化加成,K的螯合+与-OMe以及-SiEt 3从[(η 2 -COD)2 NiSiEt 3 ]是促进NiSiEt的插入的关键步骤3生成2-甲氧基萘的═C(2)碳。我们还观察到,非π扩展的芳族系统的活化能垒高于π扩展的芳族系统的活化能垒。整个研究该清单的Ni(COD)2催化的本位2-甲氧基萘的-silylation通过内部的亲核取代途径起作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号