当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Potassium fluoride activation for the nucleophilic fluorination reaction using 18-crown-6, [2.2.2]-cryptand, pentaethylene glycol and comparison with the new hydro-crown scaffold: a theoretical analysis†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1039/c8ob00418h Josefredo R. Pliego 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1039/c8ob00418h Josefredo R. Pliego 1, 2, 3, 4
Affiliation
|
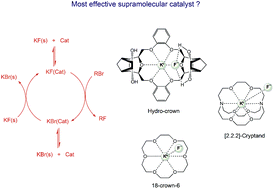
Activation of potassium fluoride salt for selective and fast nucleophilic fluorination requires its solubilization and stabilization of the respective transition state. This goal can be achieved through control of the nano-environment around the reactants via cation or ion-pair binding catalysis. In this work, six different species were theoretically investigated as promoters and catalysts for nucleophilic fluorination: tri-tert-butanolamine, 18-crown-6, pentaethylene glycol, [2.2.2]-cryptand, and two new hydroxylated crown ethers (hydro-crowns). Calculations using the PBE functional and the LPNO-CEPA method, as well as the SMD continuum model, were carried out for the SN2 reaction of KF with ethyl bromide in toluene solution as a model system. The present study points out that [2.2.2]-cryptand is the most effective promoter of the reaction when using stoichiometric quantities. In the case of a catalytic process, the new DB18C6-4OH is the most effective molecule considering only a 1 : 1 complex. The hydroxyl groups are important for the solubilization of potassium fluoride and for the catalytic cycle. Nevertheless, the DB18C6-4OH hydro-crown can form a 2 : 2 complex and is needed to add bulk groups close to the hydroxyls to avoid dimerization. The calculated overall free energy of activation for reactions promoted by 18-crown-6, pentaethylene glycol, and [2.2.2]-cryptand is in good agreement with the experimental data.
中文翻译:

氟化钾激活用于使用所述亲核氟化反应18-冠-6,[2,2,2] -cryptand,五甘醇和与新的水力冠状构架比较:理论分析†
活化氟化钾盐以进行选择性和快速的亲核氟化反应需要其溶解和稳定相应的过渡态。可以通过阳离子或离子对结合催化作用控制反应物周围的纳米环境来实现这一目标。在这项工作中,理论上研究了六种不同的物质作为亲核氟化反应的促进剂和催化剂:三叔丁醇胺,18-冠-6,五甘醇,[2.2.2] -cryptand和两种新的羟基化冠醚(氢-冠)。使用SBE函数和LPNO-CEPA方法以及SMD连续模型对S N进行了计算2 KF与溴乙烷在甲苯溶液中的反应作为模型系统。本研究指出,当使用化学计量量时,[2.2.2] -cryptand是反应的最有效启动子。在催化过程中,仅考虑1:1的配合物,新型DB18C6-4OH是最有效的分子。羟基对于氟化钾的增溶和催化循环很重要。但是,DB18C6-4OH氢冠可以形成2:2的配合物,需要添加靠近羟基的本体基团以避免二聚。计算出的由18-crown-6,五甘醇和[2.2.2] -cryptand促进的反应的总活化活化能与实验数据非常吻合。
更新日期:2018-03-16
中文翻译:

氟化钾激活用于使用所述亲核氟化反应18-冠-6,[2,2,2] -cryptand,五甘醇和与新的水力冠状构架比较:理论分析†
活化氟化钾盐以进行选择性和快速的亲核氟化反应需要其溶解和稳定相应的过渡态。可以通过阳离子或离子对结合催化作用控制反应物周围的纳米环境来实现这一目标。在这项工作中,理论上研究了六种不同的物质作为亲核氟化反应的促进剂和催化剂:三叔丁醇胺,18-冠-6,五甘醇,[2.2.2] -cryptand和两种新的羟基化冠醚(氢-冠)。使用SBE函数和LPNO-CEPA方法以及SMD连续模型对S N进行了计算2 KF与溴乙烷在甲苯溶液中的反应作为模型系统。本研究指出,当使用化学计量量时,[2.2.2] -cryptand是反应的最有效启动子。在催化过程中,仅考虑1:1的配合物,新型DB18C6-4OH是最有效的分子。羟基对于氟化钾的增溶和催化循环很重要。但是,DB18C6-4OH氢冠可以形成2:2的配合物,需要添加靠近羟基的本体基团以避免二聚。计算出的由18-crown-6,五甘醇和[2.2.2] -cryptand促进的反应的总活化活化能与实验数据非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号