当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubilities of Isophthalic Acid in Ternary Mixtures of Acetic Acid + Water + Benzoic Acid from 292.55 to 372.10 K
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.jced.7b00958 Shuyu Ning 1 , Weifeng Chen 1 , Teng Pan 1 , Youwei Cheng 1 , Lijun Wang 1 , Xi Li 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.jced.7b00958 Shuyu Ning 1 , Weifeng Chen 1 , Teng Pan 1 , Youwei Cheng 1 , Lijun Wang 1 , Xi Li 1
Affiliation
|
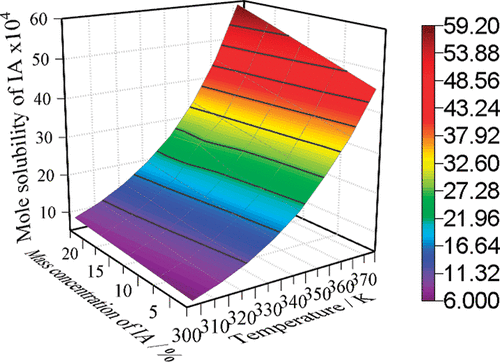
Solubilities of isophthalic acid in ternary mixtures of acetic acid + water + benzoic acid were determined by the static analytical method. The temperature range was from 292.55 to 372.10 K, the mass ratios of water to acetic acid were 0, 1:9, and 1:4, and the mass fractions of benzoic acid in initial solvent ranged from 0 to 20.1%. The experimental results indicated that the solubility of isophthalic acid increased with the introduction of benzoic acid to the binary solvents of acetic acid and water, and that could be explained by Hansen’s three-dimensional parameters and like-dissolves-like rule. Then the experimental solubilities were correlated by the nonrandom two liquids (NRTL) model, and the model parameters were regressed. The values calculated by the NRTL model showed good agreement with the experiment data.
中文翻译:

乙酸+水+苯甲酸三元混合物中间苯二甲酸在292.55至372.10 K中的溶解度。
用静态分析法测定间苯二甲酸在乙酸+水+苯甲酸三元混合物中的溶解度。温度范围为292.55至372.10K,水与乙酸的质量比为0、1:9和1:4,初始溶剂中苯甲酸的质量分数为0至20.1%。实验结果表明,间苯二甲酸的溶解度随苯甲酸向乙酸和水的二元溶剂中的引入而增加,这可以用汉森的三维参数和类似溶解类似的规律来解释。然后通过非随机两种液体(NRTL)模型对实验溶解度进行关联,并对模型参数进行回归。NRTL模型计算的值与实验数据显示出良好的一致性。
更新日期:2018-03-16
中文翻译:

乙酸+水+苯甲酸三元混合物中间苯二甲酸在292.55至372.10 K中的溶解度。
用静态分析法测定间苯二甲酸在乙酸+水+苯甲酸三元混合物中的溶解度。温度范围为292.55至372.10K,水与乙酸的质量比为0、1:9和1:4,初始溶剂中苯甲酸的质量分数为0至20.1%。实验结果表明,间苯二甲酸的溶解度随苯甲酸向乙酸和水的二元溶剂中的引入而增加,这可以用汉森的三维参数和类似溶解类似的规律来解释。然后通过非随机两种液体(NRTL)模型对实验溶解度进行关联,并对模型参数进行回归。NRTL模型计算的值与实验数据显示出良好的一致性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号