当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Bioactive 3,4-Dihydro-2H-naphtho[2,3-b][1,4]oxazine-5,10-dione and 2,3,4,5-Tetrahydro-1H-naphtho[2,3-b]azepine-6,11-dione Derivatives via the Copper-Catalyzed Intramolecular Coupling Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.joc.8b00020 Hengyao You 1 , Srinivas Rao Vegi 1 , Chandraiah Lagishetti 1 , Shuo Chen 1 , R. Santhosh Reddy 1 , Xiaohong Yang 1 , Jian Guo 1 , Chenhui Wang 1 , Yun He 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.joc.8b00020 Hengyao You 1 , Srinivas Rao Vegi 1 , Chandraiah Lagishetti 1 , Shuo Chen 1 , R. Santhosh Reddy 1 , Xiaohong Yang 1 , Jian Guo 1 , Chenhui Wang 1 , Yun He 1
Affiliation
|
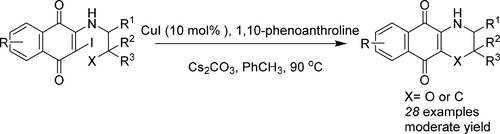
A convenient synthesis of 3,4-dihydro-2H-naphtho[2,3-b][1,4]oxazine-5,10-diones and 2,3,4,5-tetrahydro-1H-naphtho[2,3-b]azepine-6,11-diones via the copper-catalyzed intramolecular C–O/C–C coupling reaction is described. This method showed a good tolerance for functional groups and was applied to the synthesis of natural product core structures. Some coupling products exhibited moderate activities against lung cancer A549 cells.
中文翻译:

具有生物活性的3,4-二氢-2 H-萘[2,3- b ] [1,4]恶嗪-5,10-二酮和2,3,4,5-四氢-1 H-萘[2,铜催化的分子内偶联反应生成3- b ]氮杂6,11-二酮衍生物
3,4-二氢-2 H-萘[2,3- b ] [1,4]恶嗪-5,10-二酮和2,3,4,5-四氢-1 H-萘[2 ]的简便合成通过铜催化的分子内C-O / C-C偶联反应得到了3-,3 - b ]氮杂-6,11-二酮。该方法对官能团显示出良好的耐受性,并被用于天然产物核心结构的合成。一些偶联产物表现出对肺癌A549细胞的中等活性。
更新日期:2018-03-16
中文翻译:

具有生物活性的3,4-二氢-2 H-萘[2,3- b ] [1,4]恶嗪-5,10-二酮和2,3,4,5-四氢-1 H-萘[2,铜催化的分子内偶联反应生成3- b ]氮杂6,11-二酮衍生物
3,4-二氢-2 H-萘[2,3- b ] [1,4]恶嗪-5,10-二酮和2,3,4,5-四氢-1 H-萘[2 ]的简便合成通过铜催化的分子内C-O / C-C偶联反应得到了3-,3 - b ]氮杂-6,11-二酮。该方法对官能团显示出良好的耐受性,并被用于天然产物核心结构的合成。一些偶联产物表现出对肺癌A549细胞的中等活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号