当前位置:
X-MOL 学术
›
J. Hepatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantification of HBV core antibodies may help predict HBV reactivation in lymphoma patients with resolved HBV infection
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.jhep.2018.02.033 Hung-Chih Yang , Hsiao-Hui Tsou , Sung-Nan Pei , Cheng-Shyong Chang , Jia-Hong Chen , Ming Yao , Shyh-Jer Lin , Johnson Lin , Quan Yuan , Ningshao Xia , Tsang-Wu Liu , Pei-Jer Chen , Ann-Lii Cheng , Chiun Hsu
Journal of Hepatology ( IF 26.8 ) Pub Date : 2018-08-01 , DOI: 10.1016/j.jhep.2018.02.033 Hung-Chih Yang , Hsiao-Hui Tsou , Sung-Nan Pei , Cheng-Shyong Chang , Jia-Hong Chen , Ming Yao , Shyh-Jer Lin , Johnson Lin , Quan Yuan , Ningshao Xia , Tsang-Wu Liu , Pei-Jer Chen , Ann-Lii Cheng , Chiun Hsu
|
BACKGROUND & AIMS
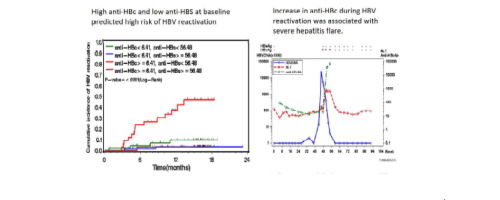
Absence or low anti-HBV surface antibody (anti-HBs) is associated with an increased risk of HBV reactivation in patients with lymphoma and resolved HBV infection receiving rituximab-containing chemotherapy. Quantification of anti-HBV core antibody (anti-HBc) is a new marker associated with the natural history and treatment response of chronic HBV infection. This study investigated whether baseline anti-HBc and anti-HBs levels may better predict HBV reactivation. METHODS
We prospectively measured the HBV DNA levels of patients with lymphoma and resolved HBV infection receiving rituximab-cyclophosphamide, hydroxydaunorubicin, vincristine, and prednisolone-based chemotherapy and started an antiviral therapy upon HBV reactivation, defined as a greater than 10-fold increase in HBV DNA compared with previous nadir levels. Anti-HBs and anti-HBc were quantified by a double-sandwich assay. Receiver-operating-characteristic-curve analysis was used to determine the optimal baseline anti-HBc/anti-HBs levels for predicting HBV reactivation. RESULTS
HBV reactivation occurred in 24 of the 197 patients enrolled, with an incidence of 11.6/100 person-years. For the 192 patients with enough serum samples for analysis, low anti-HBs (<56.48 mIU/ml) and high anti-HBc (≥6.41 IU/ml) at baseline were significantly associated with high risk of HBV reactivation (hazard ratio [HR] 8.48 and 4.52, respectively; p <0.01). The multivariate analysis indicated that (1) patients with both high anti-HBc and low anti-HBs at baseline (36 of 192 patients) had an HR of 17.29 for HBV reactivation (95% CI 3.92-76.30; p <0.001), and (2) HBV reactivation may be associated with inferior overall survival (HR 2.41; 95% CI 1.15-5.05; p = 0.02). CONCLUSIONS
Baseline anti-HBc/anti-HBs levels may predict HBV reactivation in these patients with lymphoma and help optimize prophylactic antiviral therapy for high-risk patients. LAY SUMMARY
In this study, we identified a subgroup of patients with lymphoma and resolved hepatitis B virus infection that had a high risk of hepatitis B virus reactivation after receiving rituximab-containing chemotherapy. These findings will help optimize a preventive strategy, especially in hepatitis B virus endemic regions with limited healthcare resources. Clinical trial number: NCT 00931229.
中文翻译:

HBV核心抗体的定量可能有助于预测HBV感染已解决的淋巴瘤患者的HBV再激活
背景和目的 抗 HBV 表面抗体 (anti-HBs) 的缺失或低水平与接受含利妥昔单抗化疗的淋巴瘤和已解决的 HBV 感染患者的 HBV 再激活风险增加有关。抗HBV核心抗体(anti-HBc)的定量是与慢性HBV感染的自然病程和治疗反应相关的新标志物。这项研究调查了基线抗 HBc 和抗 HBs 水平是否可以更好地预测 HBV 再激活。方法 我们前瞻性地测量了接受利妥昔单抗-环磷酰胺、羟基柔红霉素、长春新碱和泼尼松龙为基础的化疗的淋巴瘤和治愈的 HBV 感染患者的 HBV DNA 水平,并在 HBV 再激活时开始抗病毒治疗,定义为 HBV 增加超过 10 倍DNA 与之前的最低点水平相比。抗-HBs 和抗-HBc 通过双夹心法定量。接受者操作特征曲线分析用于确定预测 HBV 再激活的最佳基线抗 HBc/抗 HBs 水平。结果 纳入的 197 名患者中有 24 名发生 HBV 再激活,发生率为 11.6/100 人年。对于有足够血清样本进行分析的 192 名患者,基线时低抗 HBs (<56.48 mIU/ml) 和高抗 HBc (≥6.41 IU/ml) 与 HBV 再激活的高风险显着相关(风险比 [HR ] 分别为 8.48 和 4.52;p < 0.01)。多变量分析表明 (1) 基线时同时具有高抗 HBc 和低抗 HBs 的患者(192 名患者中的 36 名)的 HBV 再激活 HR 为 17.29(95% CI 3.92-76.30;p <0.001),(2) HBV 再激活可能与较差的总生存期有关(HR 2.41;95% CI 1.15-5.05;p = 0.02)。结论 基线抗 HBc/抗 HBs 水平可以预测这些淋巴瘤患者的 HBV 再激活,并有助于优化高危患者的预防性抗病毒治疗。常规总结 在本研究中,我们确定了一个接受含利妥昔单抗化疗后乙型肝炎病毒再激活风险高的淋巴瘤和乙型肝炎病毒感染治愈的患者亚组。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。结论 基线抗 HBc/抗 HBs 水平可以预测这些淋巴瘤患者的 HBV 再激活,并有助于优化高危患者的预防性抗病毒治疗。常规总结 在本研究中,我们确定了一个接受含利妥昔单抗化疗后乙型肝炎病毒再激活风险高的淋巴瘤和乙型肝炎病毒感染治愈的患者亚组。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。结论 基线抗 HBc/抗 HBs 水平可以预测这些淋巴瘤患者的 HBV 再激活,并有助于优化高危患者的预防性抗病毒治疗。常规总结 在本研究中,我们确定了一个接受含利妥昔单抗化疗后乙型肝炎病毒再激活风险高的淋巴瘤和乙型肝炎病毒感染治愈的患者亚组。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。我们确定了一个患有淋巴瘤并治愈了乙型肝炎病毒感染的患者亚组,这些患者在接受含利妥昔单抗的化疗后乙型肝炎病毒再激活的风险很高。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。我们确定了一个患有淋巴瘤并治愈了乙型肝炎病毒感染的患者亚组,这些患者在接受含利妥昔单抗的化疗后乙型肝炎病毒再激活的风险很高。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。
更新日期:2018-08-01
中文翻译:

HBV核心抗体的定量可能有助于预测HBV感染已解决的淋巴瘤患者的HBV再激活
背景和目的 抗 HBV 表面抗体 (anti-HBs) 的缺失或低水平与接受含利妥昔单抗化疗的淋巴瘤和已解决的 HBV 感染患者的 HBV 再激活风险增加有关。抗HBV核心抗体(anti-HBc)的定量是与慢性HBV感染的自然病程和治疗反应相关的新标志物。这项研究调查了基线抗 HBc 和抗 HBs 水平是否可以更好地预测 HBV 再激活。方法 我们前瞻性地测量了接受利妥昔单抗-环磷酰胺、羟基柔红霉素、长春新碱和泼尼松龙为基础的化疗的淋巴瘤和治愈的 HBV 感染患者的 HBV DNA 水平,并在 HBV 再激活时开始抗病毒治疗,定义为 HBV 增加超过 10 倍DNA 与之前的最低点水平相比。抗-HBs 和抗-HBc 通过双夹心法定量。接受者操作特征曲线分析用于确定预测 HBV 再激活的最佳基线抗 HBc/抗 HBs 水平。结果 纳入的 197 名患者中有 24 名发生 HBV 再激活,发生率为 11.6/100 人年。对于有足够血清样本进行分析的 192 名患者,基线时低抗 HBs (<56.48 mIU/ml) 和高抗 HBc (≥6.41 IU/ml) 与 HBV 再激活的高风险显着相关(风险比 [HR ] 分别为 8.48 和 4.52;p < 0.01)。多变量分析表明 (1) 基线时同时具有高抗 HBc 和低抗 HBs 的患者(192 名患者中的 36 名)的 HBV 再激活 HR 为 17.29(95% CI 3.92-76.30;p <0.001),(2) HBV 再激活可能与较差的总生存期有关(HR 2.41;95% CI 1.15-5.05;p = 0.02)。结论 基线抗 HBc/抗 HBs 水平可以预测这些淋巴瘤患者的 HBV 再激活,并有助于优化高危患者的预防性抗病毒治疗。常规总结 在本研究中,我们确定了一个接受含利妥昔单抗化疗后乙型肝炎病毒再激活风险高的淋巴瘤和乙型肝炎病毒感染治愈的患者亚组。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。结论 基线抗 HBc/抗 HBs 水平可以预测这些淋巴瘤患者的 HBV 再激活,并有助于优化高危患者的预防性抗病毒治疗。常规总结 在本研究中,我们确定了一个接受含利妥昔单抗化疗后乙型肝炎病毒再激活风险高的淋巴瘤和乙型肝炎病毒感染治愈的患者亚组。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。结论 基线抗 HBc/抗 HBs 水平可以预测这些淋巴瘤患者的 HBV 再激活,并有助于优化高危患者的预防性抗病毒治疗。常规总结 在本研究中,我们确定了一个接受含利妥昔单抗化疗后乙型肝炎病毒再激活风险高的淋巴瘤和乙型肝炎病毒感染治愈的患者亚组。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。我们确定了一个患有淋巴瘤并治愈了乙型肝炎病毒感染的患者亚组,这些患者在接受含利妥昔单抗的化疗后乙型肝炎病毒再激活的风险很高。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。我们确定了一个患有淋巴瘤并治愈了乙型肝炎病毒感染的患者亚组,这些患者在接受含利妥昔单抗的化疗后乙型肝炎病毒再激活的风险很高。这些发现将有助于优化预防策略,尤其是在医疗资源有限的乙型肝炎病毒流行地区。临床试验编号:NCT 00931229。











































 京公网安备 11010802027423号
京公网安备 11010802027423号