Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2018-03-16 , DOI: 10.1016/j.bmc.2018.03.008 Tomi K. Sawyer , Anthony W. Partridge , Hung Yi Kristal Kaan , Yu-Chi Juang , Shuhui Lim , Charles Johannes , Tsz Ying Yuen , Chandra Verma , Srinivasaraghavan Kannan , Pietro Aronica , Yaw Sing Tan , Brad Sherborne , Sookhee Ha , Jerome Hochman , Shiying Chen , Laura Surdi , Andrea Peier , Berengere Sauvagnat , Peter J. Dandliker , Christopher J. Brown , Simon Ng , Fernando Ferrer , David P. Lane
|
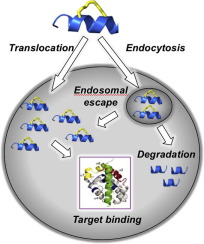
Macrocyclic α-helical peptides have emerged as a compelling new therapeutic modality to tackle targets confined to the intracellular compartment. Within the scope of hydrocarbon-stapling there has been significant progress to date, including the first stapled α-helical peptide to enter into clinical trials. The principal design concept of stapled α-helical peptides is to mimic a cognate (protein) ligand relative to binding its target via an α-helical interface. However, it was the proclivity of such stapled α-helical peptides to exhibit cell permeability and proteolytic stability that underscored their promise as unique macrocyclic peptide drugs for intracellular targets. This perspective highlights key learnings as well as challenges in basic research with respect to structure-based design, innovative chemistry, cell permeability and proteolytic stability that are essential to fulfill the promise of stapled α-helical peptide drug development.
中文翻译:

大环α螺旋肽的治疗方式:学习和挑战的角度
大环α-螺旋肽已经成为一种引人注目的新治疗手段,可以解决局限于细胞内区室的靶标。迄今为止,在碳氢化合物装订的范围内已经取得了重大进展,包括首个进入临床试验的装订的α-螺旋肽。钉合α-螺旋肽的主要设计概念是模仿同源(蛋白质)配体,使其相对于通过α-螺旋界面结合其靶标。然而,正是这种钉合的α-螺旋肽表现出细胞渗透性和蛋白水解稳定性的倾向证明了它们作为细胞内靶标独特的大环肽药物的前景。这种观点突出了基础研究,基于结构的设计,创新化学,











































 京公网安备 11010802027423号
京公网安备 11010802027423号