当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design and synthesis of imidazoles linearly connected to carbocyclic and heterocyclic rings via a 1,2,3-triazole linker. Reactivity of β-azolyl enamines towards heteroaromatic azides†
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1039/c7nj04243d Nikolai A. Beliaev 1, 2, 3 , Marsel Z. Shafikov 1, 2, 3, 4, 5 , Ilya V. Efimov 1, 2, 3 , Tetyana V. Beryozkina 1, 2, 3 , Gert Lubec 6, 7, 8, 9 , Wim Dehaen 10, 11, 12, 13, 14 , Vasiliy A. Bakulev 1, 2, 3
New Journal of Chemistry ( IF 3.3 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1039/c7nj04243d Nikolai A. Beliaev 1, 2, 3 , Marsel Z. Shafikov 1, 2, 3, 4, 5 , Ilya V. Efimov 1, 2, 3 , Tetyana V. Beryozkina 1, 2, 3 , Gert Lubec 6, 7, 8, 9 , Wim Dehaen 10, 11, 12, 13, 14 , Vasiliy A. Bakulev 1, 2, 3
Affiliation
|
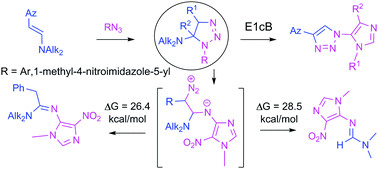
Herein we report an investigation on the 1,3-dipolar cycloaddition of 5-azido-4-nitroimidazoles to β-azolylenamines and the subsequent transformations of the intermediate 1,2,3-triazolines. 5-Azido-1-methyl-4-nitroimidazoles were combined with β-azolylenamines to form novel imidazoles linearly connected via a 1,2,3-triazole to 1,2,3-thiadiazole and isoxazole rings. On the other hand, in the reaction of β-(imidazol-4-yl)enamine with 1-methyl-4-nitro-5-azidoimidazole, the N,N-dimethyl-N′-(1-methyl-4-nitro-1H-imidazol-5-yl)formimidamide was isolated as a single product. A third type of product, active methylenes containing N-imidazol-5-yl-amidines were obtained in a similar reaction of β-phenylenamine. The factors which govern the outcome of the reactions of these heterocyclic enamines with highly electrophilic imidazolylazides were carefully studied on the basis of DFT calculations with a mPW1K density functional. The formation of various products in the reaction of imidazolyl azides with enamines was rationalized by different transformation pathways of a common 1,2,3-triazoline intermediate. According to the calculations, the two transformation paths, that are different from the path leading to aromatic 1,2,3-triazoles, occur by a stepwise mechanism involving a diazo compound formed by ring-opening of the intermediate 1,2,3-triazolines. Based on literature analysis and theoretical investigations, it is proposed that elimination of an amine from the intermediate 1,2,3-triazolines leading to 1,2,3-triazoles is a base-catalyzed process which takes place via an E1cb mechanism.
中文翻译:

通过1,2,3-三唑连接体 线性连接至碳环和杂环的咪唑的设计和合成。β-偶氮烯基烯胺对杂芳族叠氮化物的反应性†
在这里,我们报告了对5-叠氮基-4-硝基咪唑的1,3-偶极环加成反应至β-偶氮亚胺的研究,以及随后的中间体1,2,3-三唑啉的转化。将5-叠氮基-1-甲基-4-硝基咪唑与β-偶氮烯基胺结合形成新颖的咪唑,其通过1,2,3-三唑与1,2,3-噻二唑和异恶唑环线性连接。另一方面,在β-(咪唑-4-基)烯胺与1-甲基-4-硝基-5-叠氮咪唑的反应中,N,N-二甲基-N ′-(1-甲基-4-硝基)分离出作为单一产物的-1 H-咪唑-5-基)甲亚酰胺。第三类产品,含N的活性亚甲基在类似的β-亚苯基胺反应中获得-咪唑-5-基-obtained。在具有mPW1K密度泛函的DFT计算的基础上,仔细研究了控制这些杂环烯胺与高度亲电的咪唑基叠氮化物反应结果的因素。咪唑基叠氮化物与烯胺的反应中各种产物的形成通过常见的1,2,3-三唑啉中间体的不同转化途径得以合理化。根据计算,与导致芳族1,2,3-三唑的途径不同的两个转化途径是通过逐步机理发生的,该逐步机理涉及通过中间体1,2,3-的开环而形成的重氮化合物。三唑啉。根据文献分析和理论研究,建议从中间体1,2,3中除去胺,通过E1cb机制。
更新日期:2018-03-16
中文翻译:

通过1,2,3-三唑连接体 线性连接至碳环和杂环的咪唑的设计和合成。β-偶氮烯基烯胺对杂芳族叠氮化物的反应性†
在这里,我们报告了对5-叠氮基-4-硝基咪唑的1,3-偶极环加成反应至β-偶氮亚胺的研究,以及随后的中间体1,2,3-三唑啉的转化。将5-叠氮基-1-甲基-4-硝基咪唑与β-偶氮烯基胺结合形成新颖的咪唑,其通过1,2,3-三唑与1,2,3-噻二唑和异恶唑环线性连接。另一方面,在β-(咪唑-4-基)烯胺与1-甲基-4-硝基-5-叠氮咪唑的反应中,N,N-二甲基-N ′-(1-甲基-4-硝基)分离出作为单一产物的-1 H-咪唑-5-基)甲亚酰胺。第三类产品,含N的活性亚甲基在类似的β-亚苯基胺反应中获得-咪唑-5-基-obtained。在具有mPW1K密度泛函的DFT计算的基础上,仔细研究了控制这些杂环烯胺与高度亲电的咪唑基叠氮化物反应结果的因素。咪唑基叠氮化物与烯胺的反应中各种产物的形成通过常见的1,2,3-三唑啉中间体的不同转化途径得以合理化。根据计算,与导致芳族1,2,3-三唑的途径不同的两个转化途径是通过逐步机理发生的,该逐步机理涉及通过中间体1,2,3-的开环而形成的重氮化合物。三唑啉。根据文献分析和理论研究,建议从中间体1,2,3中除去胺,通过E1cb机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号