当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boron–boron, carbon–carbon and nitrogen–nitrogen bonding in N-heterocyclic carbenes and their diazaboryl and triazole analogues: Wanzlick equilibrium revisited†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1039/c8nj00296g K. Młodzikowska 1, 2, 3, 4 , A. A. Rajkiewicz 1, 2, 3, 4 , K. Grela 2, 4, 5, 6, 7 , B. Trzaskowski 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1039/c8nj00296g K. Młodzikowska 1, 2, 3, 4 , A. A. Rajkiewicz 1, 2, 3, 4 , K. Grela 2, 4, 5, 6, 7 , B. Trzaskowski 1, 2, 3, 4
Affiliation
|
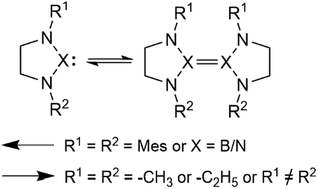
A series of monomers and dimers of N-heterocyclic carbenes and their diazaboryl and triazole analogues were investigated using the M06-2X and M06 density functional methods as well as using a DLPNO-CCSD(T) approach. We show that asymmetric carbenes bearing a benzyl and a mesityl moiety are predicted to form stable dimers due to favorable off-centre parallel stacking interactions. We also show that the double bond of the imidazole core destabilizes carbene dimers, shifting the Wanzlick equilibrium towards monomers. We predict that the methyl-, ethyl- and mesityl-substituted diazaboroles are more stable as monomers, irrespective of the presence of the double-bond in their structure, as are triazoles. Additionally, we demonstrate that the HOMO–LUMO gap difference between the monomer and the dimer, which is relatively easy to obtain, is a good indicator of the relative stability of the dimer versus the monomer for carbenes.
中文翻译:

N-杂环卡宾及其二氮杂硼烷基和三唑类似物中的硼-硼,碳-碳和氮-氮键:重新讨论了Wanzlick平衡†
使用M06-2X和M06密度泛函方法以及DLPNO-CCSD(T)方法研究了N-杂环卡宾的一系列单体和二聚体及其二氮杂硼烷基和三唑类似物。我们显示,由于有利的偏心平行堆积相互作用,预计带有苄基和异戊二烯部分的不对称碳烯形成稳定的二聚体。我们还显示,咪唑核心的双键使卡宾二聚体不稳定,将Wanzlick平衡移向单体。我们预测,甲基,乙基和异丁基取代的二氮杂硼烷作为单体更稳定,而与三唑一样,无论其结构中是否存在双键。此外,我们证明了相对容易获得的单体与二聚体之间的HOMO-LUMO间隙差异,而不是卡宾的单体。
更新日期:2018-03-16
中文翻译:

N-杂环卡宾及其二氮杂硼烷基和三唑类似物中的硼-硼,碳-碳和氮-氮键:重新讨论了Wanzlick平衡†
使用M06-2X和M06密度泛函方法以及DLPNO-CCSD(T)方法研究了N-杂环卡宾的一系列单体和二聚体及其二氮杂硼烷基和三唑类似物。我们显示,由于有利的偏心平行堆积相互作用,预计带有苄基和异戊二烯部分的不对称碳烯形成稳定的二聚体。我们还显示,咪唑核心的双键使卡宾二聚体不稳定,将Wanzlick平衡移向单体。我们预测,甲基,乙基和异丁基取代的二氮杂硼烷作为单体更稳定,而与三唑一样,无论其结构中是否存在双键。此外,我们证明了相对容易获得的单体与二聚体之间的HOMO-LUMO间隙差异,而不是卡宾的单体。











































 京公网安备 11010802027423号
京公网安备 11010802027423号