Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.jcou.2018.01.008 Kai Coenen , Fausto Gallucci , Brahim Mezari , Emiel Hensen , Martin van Sint Annaland
|
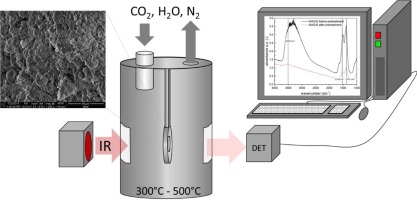
In-situ IR technique was used to study the reversible adsorption of CO2 and H2O at elevated temperatures on a potassium-promoted hydrotalcite for its use in sorption-enhanced water-gas shift (SEWGS). It was found that mainly bidentate carbonate species are responsible for the reversible (cyclic) adsorption capacity of the sorbent. The presence of H2O can enhance the decomposition of bidentate carbonates bond to the stronger basic surface-sites. The basic strength of the involved adsorption sites for bidentate formation appears to be highly heterogeneous. At higher operating temperatures, reversible formation of bulk carbonates seem to participate in the reversible adsorption for CO2. The presence of H2O on the sorbent can lead to the formation of bi-carbonate, especially at lower operating temperatures of 300 °C. The transient absorbance of the main absorption bands for carbonate species identified during this study can be used in the development of a detailed description of the reversible adsorption/desorption kinetics reported before using thermogravimetric analyses.
中文翻译:

水滑石吸附CO 2和H 2 O的原位红外研究
利用原位红外技术研究了钾促进水滑石在高温下对CO 2和H 2 O的可逆吸附,以用于吸附增强的水煤气变换(SEWGS)。已经发现,主要是二齿碳酸盐种类是造成吸附剂的可逆(循环)吸附能力的原因。H 2 O的存在可以增强双齿碳酸盐与强碱性表面部位的结合。用于二齿形成的所涉及的吸附位点的基本强度似乎是高度异质的。在较高的工作温度下,块状碳酸盐的可逆形成似乎参与了CO 2的可逆吸附。H 2的存在吸附剂上的O会导致形成碳酸氢根,尤其是在300°C的较低工作温度下。在这项研究中确定的碳酸盐种类的主要吸收带的瞬态吸收率可用于详细描述热重分析之前报道的可逆吸附/解吸动力学。









































 京公网安备 11010802027423号
京公网安备 11010802027423号