当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytochrome aa3 Oxygen Reductase Utilizes the Tunnel Observed in the Crystal Structures To Deliver O2 for Catalysis
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.biochem.7b01194 Paween Mahinthichaichan 1 , Robert B. Gennis 2 , Emad Tajkhorshid 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-03-16 00:00:00 , DOI: 10.1021/acs.biochem.7b01194 Paween Mahinthichaichan 1 , Robert B. Gennis 2 , Emad Tajkhorshid 1
Affiliation
|
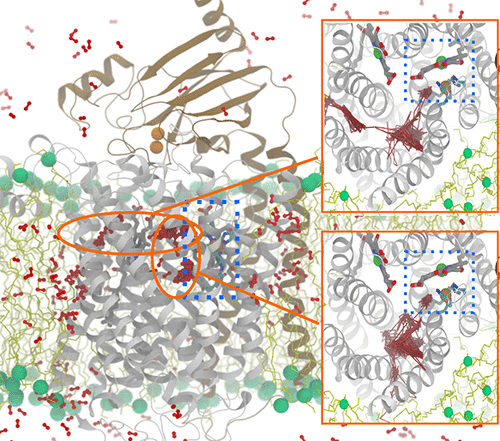
Cytochrome aa3 is the terminal respiratory enzyme of all eukaryotes and many bacteria and archaea, reducing O2 to water and harnessing the free energy from the reaction to generate the transmembrane electrochemical potential. The diffusion of O2 to the heme-copper catalytic site, which is buried deep inside the enzyme, is the initiation step of the reaction chemistry. Our previous molecular dynamics (MD) study with cytochrome ba3, a homologous enzyme of cytochrome aa3 in Thermus thermophilus, demonstrated that O2 diffuses from the lipid bilayer to its reduction site through a 25 Å long tunnel inferred by Xe binding sites detected by X-ray crystallography [Mahinthichaichan, P., Gennis, R., and Tajkhorshid, E. (2016) Biochemistry 55, 1265–1278]. Although a similar tunnel is observed in cytochrome aa3, this putative pathway appears partially occluded between the entrances and the reduction site. Also, the experimentally determined second-order rate constant for O2 delivery in cytochrome aa3 (∼108 M–1 s–1) is 10 times slower than that in cytochrome ba3 (∼109 M–1 s–1). A question to be addressed is whether cytochrome aa3 utilizes this X-ray-inferred tunnel as the primary pathway for O2 delivery. Using complementary computational methods, including multiple independent flooding MD simulations and implicit ligand sampling calculations, we probe the O2 delivery pathways in cytochrome aa3 of Rhodobacter sphaeroides. All of the O2 molecules that arrived in the reduction site during the simulations were found to diffuse through the X-ray-observed tunnel, despite its apparent constriction, supporting its role as the main O2 delivery pathway in cytochrome aa3. The rate constant for O2 delivery in cytochrome aa3, approximated using the simulation results, is 10 times slower than in cytochrome ba3, in agreement with the experimentally determined rate constants.
中文翻译:

细胞色素aa 3氧还原酶利用在晶体结构中观察到的通道传递O 2进行催化
细胞色素aa 3是所有真核生物以及许多细菌和古细菌的末端呼吸酶,可将O 2还原为水,并利用反应中的自由能产生跨膜电化学势。O 2扩散到埋藏在酶内部的血红素铜催化位点是反应化学的起始步骤。我们以前的分子动力学(MD)研究与细胞色素BA 3,细胞色素的同源酶AA 3在嗜热栖热,证明了直径:2通过X射线晶体学检测到的Xe结合位点推断出的25埃长的通道从脂质双层扩散到其还原位点[Mahinthichaichan,P.,Gennis,R.和Tajkhorshid,E.(2016)生物化学55,1265– 1278]。尽管在细胞色素aa 3中观察到类似的通道,但该假定的途径在入口和还原位点之间似乎被部分封闭。同样,实验确定的在细胞色素aa 3(〜10 8 M –1 s –1)中释放O 2的二阶速率常数比在细胞色素ba 3(〜10 9 M –1)中的二阶速率常数慢10倍。s –1)。要解决的问题是细胞色素aa 3是否利用这种X射线推断的隧道作为O 2递送的主要途径。使用补充的计算方法,包括多个独立的水淹MD模拟和隐式配体采样计算,我们在球形红球菌的细胞色素aa 3中探究了O 2传递途径。在模拟过程中,所有到达还原位点的O 2分子都被发现通过X射线观察的隧道扩散,尽管存在明显的收缩,支持了其作为细胞色素a中主要O 2传递途径的作用。3。使用模拟结果近似得出,在细胞色素aa 3中递送O 2的速率常数比细胞色素ba 3中的速率慢10倍,这与实验确定的速率常数相符。
更新日期:2018-03-16
中文翻译:

细胞色素aa 3氧还原酶利用在晶体结构中观察到的通道传递O 2进行催化
细胞色素aa 3是所有真核生物以及许多细菌和古细菌的末端呼吸酶,可将O 2还原为水,并利用反应中的自由能产生跨膜电化学势。O 2扩散到埋藏在酶内部的血红素铜催化位点是反应化学的起始步骤。我们以前的分子动力学(MD)研究与细胞色素BA 3,细胞色素的同源酶AA 3在嗜热栖热,证明了直径:2通过X射线晶体学检测到的Xe结合位点推断出的25埃长的通道从脂质双层扩散到其还原位点[Mahinthichaichan,P.,Gennis,R.和Tajkhorshid,E.(2016)生物化学55,1265– 1278]。尽管在细胞色素aa 3中观察到类似的通道,但该假定的途径在入口和还原位点之间似乎被部分封闭。同样,实验确定的在细胞色素aa 3(〜10 8 M –1 s –1)中释放O 2的二阶速率常数比在细胞色素ba 3(〜10 9 M –1)中的二阶速率常数慢10倍。s –1)。要解决的问题是细胞色素aa 3是否利用这种X射线推断的隧道作为O 2递送的主要途径。使用补充的计算方法,包括多个独立的水淹MD模拟和隐式配体采样计算,我们在球形红球菌的细胞色素aa 3中探究了O 2传递途径。在模拟过程中,所有到达还原位点的O 2分子都被发现通过X射线观察的隧道扩散,尽管存在明显的收缩,支持了其作为细胞色素a中主要O 2传递途径的作用。3。使用模拟结果近似得出,在细胞色素aa 3中递送O 2的速率常数比细胞色素ba 3中的速率慢10倍,这与实验确定的速率常数相符。











































 京公网安备 11010802027423号
京公网安备 11010802027423号