Journal of Solid State Chemistry ( IF 3.2 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.jssc.2018.03.005 Sarah Abduljabbar Yaseen , Ghadah Abdaljabar Yiseen , Zongjin Li
|
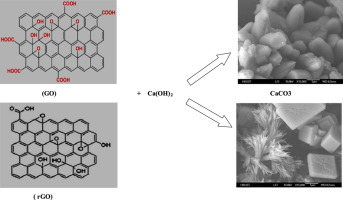
This paper reports a new approach of producing CaCO3 particles in alkali solution. CaCO3 particles with pure calcite structure were obtained from the reaction of water-dispersed graphene oxide (GO) or reduced graphene oxide (rGO) with either Ca(OH)2 or CaO. In Fourier Transform Infrared (FTIR) spectra, the pure calcite structure was demonstrated by fundamental bands at 1425 (ν3), 873 (ν2), and 712 cm−1 (ν4). The Raman spectra showed the characteristic peak of calcite structure at 1085 cm−1 (ν1). X-ray diffraction pattern (XRD) and X-ray photoelectron spectroscopy (XPS) analyses further confirmed that only the pure calcite phase of CaCO3 was formed in both synthesis approaches. Scanning electron microscopy (SEM), Energy dispersive X-ray analyzer (EDX), and High-resolution transmission electron microscopy (HRTEM) also confirmed that distorted cubic and rhombic calcite particles were obtained with GO, while the pine flower-like and flower-like particles were obtained with rGO, and the average crystallite sizes varied from 26–44 nm. The mechanism of the reaction was investigated and it was found that the decomposition of oxygen functional groups on the surface of GO or rGO in certain alkaline media to release CO, CO2, and water was a key process as the released CO2 further reacted with OH- and Ca2+ to form CaCO3. This demonstrated that both GO and rGO could be used as main reactants for the synthesis of calcite.
中文翻译:

氧化石墨烯和还原氧化石墨烯在碱溶液中合成碳酸钙
本文报道了一种在碱溶液中生产CaCO 3颗粒的新方法。从水分散的氧化石墨烯(GO)或还原的氧化石墨烯(rGO)与Ca(OH)2或CaO的反应中获得具有纯方解石结构的CaCO 3颗粒。在傅里叶变换红外光谱(FTIR),纯方解石结构由基本频带在1425证实( ν3),873(ν2),和712厘米-1(ν4)。拉曼光谱在1085cm -1(ν1)处显示方解石结构的特征峰。X射线衍射图谱(XRD)和X射线光电子能谱(XPS)分析进一步证实,只有纯方解石相的CaCO 3在两种合成方法中均形成。扫描电子显微镜(SEM),能量色散X射线分析仪(EDX)和高分辨率透射电子显微镜(HRTEM)也证实,GO可以得到扭曲的方解石和菱形方解石颗粒,而松花状和花状用rGO获得了类似的颗粒,平均微晶尺寸在26–44 nm之间变化。研究了反应机理,发现在某些碱性介质中GO或rGO表面的氧官能团的分解释放出CO,CO 2和水是关键的过程,因为释放出的CO 2进一步与CO 2反应。 OH -和Ca 2+形成的CaCO 3。这表明GO和rGO都可以用作方解石合成的主要反应物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号