当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Use of a tyrosine analog to modulate the two activities of a non-heme iron enzyme OvoA in ovothiol biosynthesis, cysteine oxidation versus oxidative C-S bond formation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-15 , DOI: 10.1021/jacs.7b13628 Li Chen 1, 2 , Nathchar Naowarojna 2 , Heng Song 2, 3 , Shu Wang 2 , Jiangyun Wang 4 , Zixin Deng 1 , Changming Zhao 1, 2 , Pinghua Liu 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-15 , DOI: 10.1021/jacs.7b13628 Li Chen 1, 2 , Nathchar Naowarojna 2 , Heng Song 2, 3 , Shu Wang 2 , Jiangyun Wang 4 , Zixin Deng 1 , Changming Zhao 1, 2 , Pinghua Liu 2
Affiliation
|
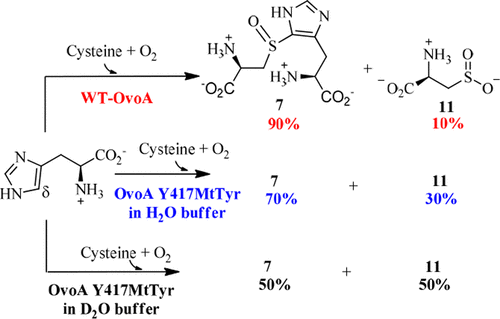
Ovothiol is a histidine thiol derivative. The biosynthesis of ovothiol involves an extremely efficient trans-sulfuration strategy. The nonheme iron enzyme OvoA catalyzed oxidative coupling between cysteine and histidine is one of the key steps. Besides catalyzing the oxidative coupling between cysteine and histidine, OvoA also catalyzes the oxidation of cysteine to cysteine sulfinic acid (cysteine dioxygenase activity). Thus far, very little mechanistic information is available for OvoA-catalysis. In this report, we measured the kinetic isotope effect (KIE) in OvoA-catalysis using the isotopically sensitive branching method. In addition, by replacing an active site tyrosine (Tyr417) with 2-amino-3-(4-hydroxy-3-(methylthio)phenyl)propanoic acid (MtTyr) through the amber suppressor mediated unnatural amino acid incorporation method, the two OvoA activities (oxidative coupling between cysteine and histidine, and cysteine dioxygenase activity) can be modulated. These results suggest that the two OvoA activities branch out from a common intermediate and that the active site tyrosine residue plays some key roles in controlling the partitioning between these two pathways.
中文翻译:

使用酪氨酸类似物调节卵硫醇生物合成中非血红素铁酶 OvoA 的两种活性,半胱氨酸氧化与氧化 CS 键形成
Ovothiol 是一种组氨酸硫醇衍生物。卵硫醇的生物合成涉及极其有效的反式硫化策略。非血红素铁酶 OvoA 催化半胱氨酸和组氨酸之间的氧化偶联是关键步骤之一。除了催化半胱氨酸和组氨酸之间的氧化偶联外,OvoA 还催化半胱氨酸氧化成半胱氨酸亚磺酸(半胱氨酸双加氧酶活性)。迄今为止,关于 OvoA 催化的机制信息非常少。在本报告中,我们使用同位素敏感分支方法测量了 OvoA 催化中的动力学同位素效应 (KIE)。此外,通过琥珀抑制子介导的非天然氨基酸掺入方法,将活性位点酪氨酸(Tyr417)替换为2-氨基-3-(4-羟基-3-(甲硫基)苯基)丙酸(MtTyr),这两个OvoA活性(半胱氨酸和组氨酸之间的氧化偶联,以及半胱氨酸双加氧酶活性)可以被调节。这些结果表明,两种 OvoA 活性从一个共同的中间体分支出来,并且活性位点酪氨酸残基在控制这两种途径之间的分配中发挥着一些关键作用。
更新日期:2018-03-15
中文翻译:

使用酪氨酸类似物调节卵硫醇生物合成中非血红素铁酶 OvoA 的两种活性,半胱氨酸氧化与氧化 CS 键形成
Ovothiol 是一种组氨酸硫醇衍生物。卵硫醇的生物合成涉及极其有效的反式硫化策略。非血红素铁酶 OvoA 催化半胱氨酸和组氨酸之间的氧化偶联是关键步骤之一。除了催化半胱氨酸和组氨酸之间的氧化偶联外,OvoA 还催化半胱氨酸氧化成半胱氨酸亚磺酸(半胱氨酸双加氧酶活性)。迄今为止,关于 OvoA 催化的机制信息非常少。在本报告中,我们使用同位素敏感分支方法测量了 OvoA 催化中的动力学同位素效应 (KIE)。此外,通过琥珀抑制子介导的非天然氨基酸掺入方法,将活性位点酪氨酸(Tyr417)替换为2-氨基-3-(4-羟基-3-(甲硫基)苯基)丙酸(MtTyr),这两个OvoA活性(半胱氨酸和组氨酸之间的氧化偶联,以及半胱氨酸双加氧酶活性)可以被调节。这些结果表明,两种 OvoA 活性从一个共同的中间体分支出来,并且活性位点酪氨酸残基在控制这两种途径之间的分配中发挥着一些关键作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号