当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Enantioselective Pyridone C-H Functionalizations Enabled by a Bulky N-Heterocyclic Carbene Ligand
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-15 , DOI: 10.1021/jacs.8b01181 Johannes Diesel 1 , Anastasiia M. Finogenova 1 , Nicolai Cramer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-15 , DOI: 10.1021/jacs.8b01181 Johannes Diesel 1 , Anastasiia M. Finogenova 1 , Nicolai Cramer 1
Affiliation
|
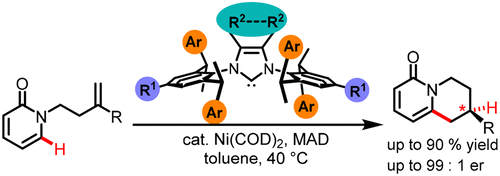
Annulated pyridones are an important scaffold found in many biologically active compounds. A Ni(0)-catalyzed C-H functionalization of 2- and 4-pyridones is disclosed, providing access to annulated pyridones via enantioselective intramolecular olefin hydroarylation. Key to the success of the transformation was the development of a sterically hindered and tunable N-heterocyclic carbene ligand resembling a chiral version of IPr. This ligand allows for mild reaction temperatures, and leads to the annulated pyridones in excellent yields and enantioselectivities.
中文翻译:

镍催化的对映选择性吡啶酮 CH 官能化由大块 N-杂环卡宾配体实现
环状吡啶酮是在许多生物活性化合物中发现的重要支架。公开了 Ni(0) 催化的 2-和 4-吡啶酮的 CH 官能化,通过对映选择性分子内烯烃加氢芳基化提供了对环状吡啶酮的访问。转化成功的关键是开发了空间位阻可调的 N-杂环卡宾配体,类似于 IPr 的手性版本。该配体允许温和的反应温度,并以优异的产率和对映选择性产生环化吡啶酮。
更新日期:2018-03-15
中文翻译:

镍催化的对映选择性吡啶酮 CH 官能化由大块 N-杂环卡宾配体实现
环状吡啶酮是在许多生物活性化合物中发现的重要支架。公开了 Ni(0) 催化的 2-和 4-吡啶酮的 CH 官能化,通过对映选择性分子内烯烃加氢芳基化提供了对环状吡啶酮的访问。转化成功的关键是开发了空间位阻可调的 N-杂环卡宾配体,类似于 IPr 的手性版本。该配体允许温和的反应温度,并以优异的产率和对映选择性产生环化吡啶酮。











































 京公网安备 11010802027423号
京公网安备 11010802027423号