当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalysis of Radical Cyclizations from Alkyl Iodides Under H2: Evidence for Electron Transfer from [CpV(CO)3H]–
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-15 , DOI: 10.1021/jacs.8b02119 Jonathan L Kuo 1 , Chris Lorenc 1 , Janine M Abuyuan 2 , Jack R Norton 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-15 , DOI: 10.1021/jacs.8b02119 Jonathan L Kuo 1 , Chris Lorenc 1 , Janine M Abuyuan 2 , Jack R Norton 1
Affiliation
|
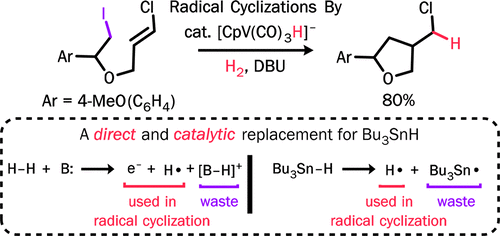
Radical cyclizations are most often achieved with Bu3SnH in the presence of a radical initiator, but environmental considerations demand that alternative reagents be developed-ones that can serve as a synthetic equivalent to the hydrogen atom. We have revisited [CpV(CO)3H]-, a known replacement for Bu3SnH, and found that it can be used catalytically under H2 in the presence of a base. We have carried out tin-free catalytic radical cyclizations of alkyl iodide substrates. The reactions are atom-efficient, and the conditions are mild, with broad tolerance for functional groups. We have, for example, achieved the first 5-exo radical cyclization involving attack onto a vinyl chloride. We suggest that the radicals are generated by an initial electron transfer.
中文翻译:

H2 下烷基碘自由基环化的催化:[CpV(CO)3H]– 电子转移的证据
自由基环化通常是在自由基引发剂存在下用 Bu3SnH 实现的,但出于环境考虑,需要开发替代试剂——可以作为氢原子的合成等价物。我们重新审视了 [CpV(CO)3H]-(Bu3SnH 的已知替代品),发现它可以在碱存在下在 H2 下起催化作用。我们已经对烷基碘底物进行了无锡催化自由基环化。该反应具有原子效率,条件温和,对官能团具有广泛的耐受性。例如,我们已经实现了第一个涉及攻击氯乙烯的 5-exo 自由基环化。我们认为自由基是由初始电子转移产生的。
更新日期:2018-03-15
中文翻译:

H2 下烷基碘自由基环化的催化:[CpV(CO)3H]– 电子转移的证据
自由基环化通常是在自由基引发剂存在下用 Bu3SnH 实现的,但出于环境考虑,需要开发替代试剂——可以作为氢原子的合成等价物。我们重新审视了 [CpV(CO)3H]-(Bu3SnH 的已知替代品),发现它可以在碱存在下在 H2 下起催化作用。我们已经对烷基碘底物进行了无锡催化自由基环化。该反应具有原子效率,条件温和,对官能团具有广泛的耐受性。例如,我们已经实现了第一个涉及攻击氯乙烯的 5-exo 自由基环化。我们认为自由基是由初始电子转移产生的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号