Chem ( IF 19.1 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.chempr.2018.02.022 Jason Y.C. Lim , Paul D. Beer
|
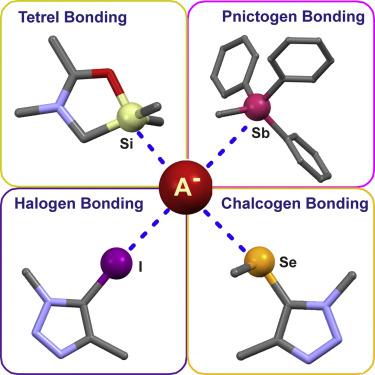
Sigma (σ)-holes are electron-deficient regions that arise from the anisotropic distribution of electron density on the atom of group 14 (tetrels), 15 (pnictogens), 16 (chalcogens), and 17 (halogens) elements when covalently bonded to electron-withdrawing groups. Named after the donor atom's group, the σ-hole interactions, halogen bonding, and chalcogen bonding with anionic species have found ground-breaking applications in anion supramolecular chemistry within the last decade. In this review, we feature key recent discoveries and advances across the whole range of σ-hole interactions for anion recognition, from the familiar halogen bonding to the almost unknown pnictogen and tetrel bonding. In particular, the novel anion recognition properties and applications that result from the unique aspects of each σ-hole interaction, together with detailed design considerations of anion-binding receptor motifs, are highlighted.
中文翻译:

阴离子识别中的Sigma-Hole相互作用
Sigma(σ)孔是缺电子的区域,当与第14组(tetrels),15个(光子),16个(硫族元素)和17个(卤素)元素的原子上的电子密度各向异性分布时,产生吸电子基团。σ-空穴相互作用,卤素键和硫属元素键与阴离子物种之间的关系以施主原子的基团命名,在过去的十年中已在阴离子超分子化学中取得了突破性的应用。在这篇综述中,我们将重点介绍在整个σ-孔相互作用范围内用于阴离子识别的最新发现和进展,从熟悉的卤素键到几乎不为人知的光原和蝶形键。尤其是,由于每个σ空穴相互作用的独特方面而产生的新颖的阴离子识别特性和应用,











































 京公网安备 11010802027423号
京公网安备 11010802027423号