Chem ( IF 19.1 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.chempr.2018.02.004 Jared M. Taylor , Patrick J. Dwyer , Joel W. Reid , Benjamin S. Gelfand , Dae-woon Lim , Masaki Donoshita , Stanislav L. Veinberg , Hiroshi Kitagawa , V. Nicholas Vukotic , George K.H. Shimizu
|
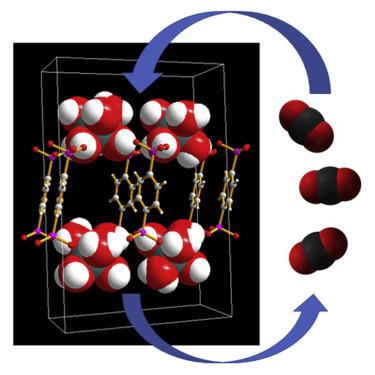
To form any porous solid requires payment of an energetic penalty to create voids through either strong bonds or many weak interactions working in concert. Here, we present a porous solid that is composed, after pore activation, of 27 wt % water and sustained by charge-assisted hydrogen bonds between hexaaquachromium(III) cations and organophosphonate anions. The network forms a pillared layered motif including guest solvents, as confirmed crystallographically. Although aquo ligands are an integral part of the hydrogen-bonding network that sustains the pores, activation under high vacuum is possible, and the network demonstrates reversible CO2 sorption. The stability of the network is attributed to the inertness of the octahedral d3 Cr(III) center in combination with high degrees of complementarity and cooperativity of hydrogen bonds. The sub-nanometer pores are guest selective on the basis of size, shape, and chemical functionality.
中文翻译:

用水保持开放的微孔:六价铬(III)阳离子支持的氢键网络
要形成任何多孔固体,都需要付出高昂的代价,才能通过牢固的键合或许多弱相互作用共同产生空隙。在这里,我们介绍了一种多孔固体,该多孔固体在孔活化后由27 wt%的水组成,并由六水合铬(III)阳离子和有机膦酸根阴离子之间的电荷辅助氢键维持。该网络形成柱状分层的基序,包括客体溶剂,如通过晶体学证实的。尽管水族配体是维持孔的氢键网络的组成部分,但在高真空下活化是可能的,并且该网络显示出可逆的CO 2吸附。网络的稳定性归因于八面体d 3的惰性Cr(III)中心与氢键的高度互补性和协同性结合在一起。根据尺寸,形状和化学功能,亚纳米孔是客体选择性的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号