当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric [3+2] Photocycloadditions of Cyclopropanes with Alkenes or Alkynes through Visible‐Light Excitation of Catalyst‐Bound Substrates
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-10 , DOI: 10.1002/anie.201802316 Xiaoqiang Huang 1 , Jiahui Lin 1 , Tianqi Shen 1 , Klaus Harms 1 , Marianna Marchini 2 , Paola Ceroni 2 , Eric Meggers 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2018-04-10 , DOI: 10.1002/anie.201802316 Xiaoqiang Huang 1 , Jiahui Lin 1 , Tianqi Shen 1 , Klaus Harms 1 , Marianna Marchini 2 , Paola Ceroni 2 , Eric Meggers 1
Affiliation
|
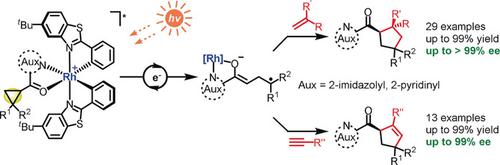
The herein reported visible‐light‐activated catalytic asymmetric [3+2] photocycloadditions between cyclopropanes and alkenes or alkynes provide access to chiral cyclopentanes and cyclopentenes, respectively, in 63–99 % yields and with excellent enantioselectivities of up to >99 % ee. The reactions are catalyzed by a single bis‐cyclometalated chiral‐at‐metal rhodium complex (2–8 mol %) which after coordination to the cyclopropane generates the visible‐light‐absorbing complex, lowers the reduction potential of the cyclopropane, and provides the asymmetric induction and overall stereocontrol. Enabled by a mild single‐electron‐transfer reduction of directly photoexcited catalyst/substrate complexes, the presented transformations expand the scope of catalytic asymmetric photocycloadditions to simple mono‐acceptor‐substituted cyclopropanes affording previously inaccessible chiral cyclopentane and cyclopentene derivatives.
中文翻译:

通过可见光激发催化剂结合的底物与烯烃或炔烃的不对称[3 + 2]环丙烷与环丙烷的光环加成反应
本文报道的环丙烷与烯烃或炔烃之间可见光激活的催化不对称[3 + 2]光环加成反应分别提供了手性环戊烷和环戊烯的产率,产率为63-99%,对映选择性高达ee高达99%以上。该反应由单一的双环金属化手性金属铑配合物(2-8 mol%)催化,在与环丙烷配位后生成可见光吸收配合物,降低了环丙烷的还原电位,并提供了不对称感应和整体立体声控制。通过直接光激发的催化剂/底物复合物的单电子转移温和还原,所提出的转化将催化不对称光环加成反应的范围扩展到简单的单受体取代的环丙烷,从而提供了以前无法获得的手性环戊烷和环戊烯衍生物。
更新日期:2018-04-10
中文翻译:

通过可见光激发催化剂结合的底物与烯烃或炔烃的不对称[3 + 2]环丙烷与环丙烷的光环加成反应
本文报道的环丙烷与烯烃或炔烃之间可见光激活的催化不对称[3 + 2]光环加成反应分别提供了手性环戊烷和环戊烯的产率,产率为63-99%,对映选择性高达ee高达99%以上。该反应由单一的双环金属化手性金属铑配合物(2-8 mol%)催化,在与环丙烷配位后生成可见光吸收配合物,降低了环丙烷的还原电位,并提供了不对称感应和整体立体声控制。通过直接光激发的催化剂/底物复合物的单电子转移温和还原,所提出的转化将催化不对称光环加成反应的范围扩展到简单的单受体取代的环丙烷,从而提供了以前无法获得的手性环戊烷和环戊烯衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号