当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C5-Morpholinomethylation of N1-sulfonylcytosines by a one-pot microwave assisted Mannich reaction†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8ob00253c Josipa Matić 1, 2, 3, 4, 5 , Irena Nekola 4, 5, 6 , Aleksandar Višnjevac 3, 4, 7, 8, 9 , Renata Kobetić 1, 2, 3, 4, 5 , Irena Martin-Kleiner 3, 4, 5, 10, 11 , Marijeta Kralj 3, 4, 5, 10, 11 , Biserka Žinić 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8ob00253c Josipa Matić 1, 2, 3, 4, 5 , Irena Nekola 4, 5, 6 , Aleksandar Višnjevac 3, 4, 7, 8, 9 , Renata Kobetić 1, 2, 3, 4, 5 , Irena Martin-Kleiner 3, 4, 5, 10, 11 , Marijeta Kralj 3, 4, 5, 10, 11 , Biserka Žinić 1, 2, 3, 4, 5
Affiliation
|
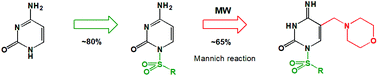
A fast and efficient route for the introduction of a methylene bridged-amine (morpholinomethyl) functionality in the C5 position of the sulfonylated cytosine nucleobase has been developed. First, novel N1-sulfonylcytosine derivatives 3–6 were prepared by the condensation of silylated cytosine with selected sulfonyl chlorides. They were subsequently transformed to 5-morpholinomethyl-N1-sulfonylcytosine derivatives (8, 12–15) using microwave irradiation. As a result of cytosine ring opening in N1-tosylcytosine, depending on the reaction conditions, peculiar tosyl-urea derivative 9 has been isolated, which provided additional insight into the reaction pathway. The influence of the C5-substituent on the antiproliferative activity has been evaluated by performing the MTT test on U251, MCF-7 and MOLT-4 tumor cell-lines.
中文翻译:

一锅微波辅助曼尼希反应 对N 1-磺酰基胞嘧啶的C5-Morpholinomethylation †
已经开发了在磺酰化胞嘧啶核苷碱基的C5位引入亚甲基桥接胺(吗啉代甲基)官能团的快速有效途径。首先,通过将甲硅烷基化的胞嘧啶与选定的磺酰氯缩合制备新的N 1-磺酰基胞嘧啶衍生物3-6。他们随后被转化成5- morpholinomethyl- Ñ 1- sulfonylcytosine衍生物(8,12-15使用微波辐射)。由于N 1-tosylcytosine中的胞嘧啶开环,根据反应条件,异戊糖脲衍生物9已分离,这提供了对反应途径的进一步了解。通过对U251,MCF-7和MOLT-4肿瘤细胞系进行MTT试验,可以评估C5取代基对抗增殖活性的影响。
更新日期:2018-03-15
中文翻译:

一锅微波辅助曼尼希反应 对N 1-磺酰基胞嘧啶的C5-Morpholinomethylation †
已经开发了在磺酰化胞嘧啶核苷碱基的C5位引入亚甲基桥接胺(吗啉代甲基)官能团的快速有效途径。首先,通过将甲硅烷基化的胞嘧啶与选定的磺酰氯缩合制备新的N 1-磺酰基胞嘧啶衍生物3-6。他们随后被转化成5- morpholinomethyl- Ñ 1- sulfonylcytosine衍生物(8,12-15使用微波辐射)。由于N 1-tosylcytosine中的胞嘧啶开环,根据反应条件,异戊糖脲衍生物9已分离,这提供了对反应途径的进一步了解。通过对U251,MCF-7和MOLT-4肿瘤细胞系进行MTT试验,可以评估C5取代基对抗增殖活性的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号