Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.cplett.2018.03.026 Irtaza Hassan , Luca Donati , Till Stensitzki , Bettina G. Keller , Karsten Heyne , Petra Imhof
|
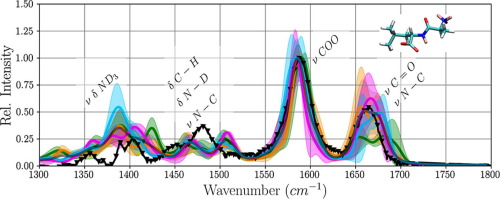
We have combined infrared (IR) experiments with molecular dynamics (MD) simulations in solution at finite temperature to analyse the vibrational signature of the small floppy peptide Alanine-Leucine. IR spectra computed from first-principles MD simulations exhibit no distinct differences between conformational clusters of α-helix or β-sheet-like folds with different orientations of the bulky leucine side chain. All computed spectra show two prominent bands, in good agreement with the experiment, that are assigned to the stretch vibrations of the carbonyl and carboxyl group, respectively. Variations in band widths and exact maxima are likely due to small fluctuations in the backbone torsion angles.
中文翻译:

从红外实验和第一性原理计算得出酰胺化区域中水合丙氨酸-亮氨酸肽的振动光谱
我们将红外(IR)实验与分子动力学(MD)模拟在有限温度下的溶液中进行了组合,以分析软盘小肽丙氨酸-亮氨酸的振动信号。从第一性原理MD模拟计算得出的红外光谱在α构象簇之间没有明显区别-螺旋或β亮氨酸侧链具有不同方向的折叠状折叠。所有计算出的光谱均显示出两个与实验非常吻合的显着谱带,分别对应于羰基和羧基的拉伸振动。带宽和精确最大值的变化可能是由于主干扭转角的微小波动引起的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号