Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.cplett.2018.03.011 Yoshiharu Mori , Hisashi Okumura , Takayoshi Watanabe , Takahiro Hohsaka
|
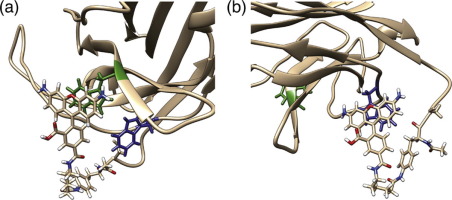
We performed metadynamics molecular dynamics simulations to reveal mechanism of antigen-dependent fluorescence response observed for site-specifically fluorescent-labeled single-chain antibody against c-Myc peptide antigen. We found that VH and VL bind with each other only when the antigen exists and that the fluorophore labeled at the N-terminus of VH interacts with Trp103 most stably. These results support the mechanism proposed from previous experiments: In the absence of antigen, Trp residues are partially exposed at the interface of VH and quench the fluorophore. In the presence of antigen, the Trp residues are buried between VH and VL, and the quenching is eliminated.
中文翻译:

分子动力学模拟研究抗c-Myc猝灭抗体的抗原依赖性荧光反应
我们进行了元动力学分子动力学模拟,以揭示针对c-Myc肽抗原的位点特异性荧光标记单链抗体所观察到的抗原依赖性荧光反应的机制。我们发现V H和V L仅当抗原存在且在V H N端标记的荧光团相互结合时与Trp103最稳定地交互。这些结果支持了先前实验提出的机制:在没有抗原的情况下,Trp残基部分暴露在V H的界面上并淬灭荧光团。在抗原存在下,Trp残基被埋在V H之间和V L,消除了淬灭。











































 京公网安备 11010802027423号
京公网安备 11010802027423号