当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bloodstream Stability Predetermines the Antitumor Efficacy of Micellar Polymer–Doxorubicin Drug Conjugates with pH-Triggered Drug Release
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00156 Petr Chytil 1 , Milada Šírová 2 , Júlia Kudláčová 1 , Blanka Říhová 2 , Karel Ulbrich 1 , Tomáš Etrych 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acs.molpharmaceut.8b00156 Petr Chytil 1 , Milada Šírová 2 , Júlia Kudláčová 1 , Blanka Říhová 2 , Karel Ulbrich 1 , Tomáš Etrych 1
Affiliation
|
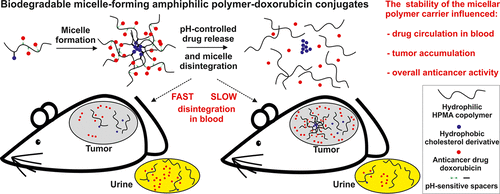
Herein, the biodegradable micelle-forming amphiphilic N-(2-hydroxypropyl) methacrylamide (HPMA)-based polymer conjugates with the anticancer drug doxorubicin (Dox) designed for enhanced tumor accumulation were investigated, and the influence of their stability in the bloodstream on biodistribution, namely, tumor uptake, and in vivo antitumor efficacy were evaluated in detail. Dox was attached to the polymer carrier by a hydrazone bond enabling pH-controlled drug release. While the polymer–drug conjugates were stable in a buffer at pH 7.4 (mimicking bloodstream environment), Dox was released in a buffer under mild acidic conditions modeling the tumor microenvironment or cells. The amphiphilic polymer carriers differed in the structure of hydrophobic cholesterol derivative moieties bound to the HPMA copolymers via a hydrolyzable hydrazone bond, exhibiting different rates of micellar structure disintegration at various pH values. Considerable dependence of the studied polymer–drug conjugate biodistribution on the stability of the micellar structure was observed in neutral, bloodstream-mimicking, environment, showing that a faster rate of the micelle disintegration in pH 7.4 increased the conjugate blood clearance, decreased tumor accumulation, and significantly reduced the tumor:blood and tumor:muscle ratios. Similarly, the final therapeutic outcome was strongly affected by the stability of the micellar structure because the most stable micellar conjugate showed an almost similar therapeutic outcome as the water-soluble, nondegradable, high-molecular-weight starlike HPMA copolymer–Dox conjugate, which was highly efficient in the treatment of solid tumors in mice. Based on the results, we conclude that the bloodstream stability of micellar polymer–anticancer drug conjugates, in addition to their low side toxicity, is a crucial parameter for their efficient solid tumor accumulation and high in vivo antitumor activity.
中文翻译:

血流稳定性决定了胶束聚合物-阿霉素药物的抗肿瘤功效与pH引发的药物释放有关
在这里,研究了可生物降解的形成胶团的两亲性N-(2-羟丙基)甲基丙烯酰胺(HPMA)聚合物共轭物与专为增强肿瘤蓄积而设计的抗癌药阿霉素(Dox),并研究了其在血流中的稳定性对生物分布的影响即肿瘤吸收和体内抗肿瘤功效进行了详细评估。Dox通过键连接到聚合物载体,从而实现pH值控制的药物释放。尽管聚合物-药物偶联物在pH 7.4(模拟血流环境)的缓冲液中稳定,但Dox在模拟肿瘤微环境或细胞的温和酸性条件下在缓冲液中释放。两亲性聚合物载体通过可水解的derivative键与HPMA共聚物结合的疏水胆固醇衍生物部分的结构不同,在各种pH值下胶束结构的分解速率不同。在中性,模仿血流的环境中,观察到所研究的聚合物-药物共轭物生物分布对胶束结构稳定性的显着依赖性,表明在pH 7时胶束崩解的速度更快。4增加了结合物的血液清除率,减少了肿瘤的积累,并显着降低了肿瘤:血液和肿瘤:肌肉的比例。同样,最终的治疗结果也受到胶束结构稳定性的强烈影响,因为最稳定的胶束结合物显示出与水溶性,不可降解,高分子量星形HPMA共聚物-Dox结合物几乎相似的治疗结果。高度有效地治疗小鼠实体瘤。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。肌肉比例。同样,最终的治疗结果也受到胶束结构稳定性的强烈影响,因为最稳定的胶束结合物显示出与水溶性,不可降解,高分子量星形HPMA共聚物-Dox结合物几乎相似的治疗结果。高度有效地治疗小鼠实体瘤。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。肌肉比例。同样,最终的治疗结果也受到胶束结构稳定性的强烈影响,因为最稳定的胶束结合物显示出与水溶性,不可降解,高分子量星形HPMA共聚物-Dox结合物几乎相似的治疗结果。高度有效地治疗小鼠实体瘤。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。高分子量星形HPMA共聚物-Dox偶联物,在治疗小鼠实体瘤方面非常有效。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。高分子量星形HPMA共聚物-Dox偶联物,在治疗小鼠实体瘤方面非常有效。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。体内抗肿瘤活性。
更新日期:2018-03-15
中文翻译:

血流稳定性决定了胶束聚合物-阿霉素药物的抗肿瘤功效与pH引发的药物释放有关
在这里,研究了可生物降解的形成胶团的两亲性N-(2-羟丙基)甲基丙烯酰胺(HPMA)聚合物共轭物与专为增强肿瘤蓄积而设计的抗癌药阿霉素(Dox),并研究了其在血流中的稳定性对生物分布的影响即肿瘤吸收和体内抗肿瘤功效进行了详细评估。Dox通过键连接到聚合物载体,从而实现pH值控制的药物释放。尽管聚合物-药物偶联物在pH 7.4(模拟血流环境)的缓冲液中稳定,但Dox在模拟肿瘤微环境或细胞的温和酸性条件下在缓冲液中释放。两亲性聚合物载体通过可水解的derivative键与HPMA共聚物结合的疏水胆固醇衍生物部分的结构不同,在各种pH值下胶束结构的分解速率不同。在中性,模仿血流的环境中,观察到所研究的聚合物-药物共轭物生物分布对胶束结构稳定性的显着依赖性,表明在pH 7时胶束崩解的速度更快。4增加了结合物的血液清除率,减少了肿瘤的积累,并显着降低了肿瘤:血液和肿瘤:肌肉的比例。同样,最终的治疗结果也受到胶束结构稳定性的强烈影响,因为最稳定的胶束结合物显示出与水溶性,不可降解,高分子量星形HPMA共聚物-Dox结合物几乎相似的治疗结果。高度有效地治疗小鼠实体瘤。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。肌肉比例。同样,最终的治疗结果也受到胶束结构稳定性的强烈影响,因为最稳定的胶束结合物显示出与水溶性,不可降解,高分子量星形HPMA共聚物-Dox结合物几乎相似的治疗结果。高度有效地治疗小鼠实体瘤。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。肌肉比例。同样,最终的治疗结果也受到胶束结构稳定性的强烈影响,因为最稳定的胶束结合物显示出与水溶性,不可降解,高分子量星形HPMA共聚物-Dox结合物几乎相似的治疗结果。高度有效地治疗小鼠实体瘤。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。高分子量星形HPMA共聚物-Dox偶联物,在治疗小鼠实体瘤方面非常有效。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。高分子量星形HPMA共聚物-Dox偶联物,在治疗小鼠实体瘤方面非常有效。根据结果,我们得出的结论是,胶束聚合物-抗癌药物偶联物的血流稳定性,除了低毒副作用外,是其有效实体瘤蓄积和高血脂的关键参数。体内抗肿瘤活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号