当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric total syntheses of callistrilones B, G and J†
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8qo00130h Li-Jun Hu 1, 2, 3, 4 , Min-Jing Cheng 4, 5, 6, 7, 8 , Jia-Qing Cao 4, 5, 6, 7 , Li-Ping Zhong 4, 8, 9, 10 , Ya-Jian Hu 4, 8, 9, 10 , Ying Wang 4, 5, 6, 7 , Lei Wang 4, 5, 6, 7 , Wen-Cai Ye 1, 2, 3, 4, 5 , Chuang-Chuang Li 4, 8, 9, 10
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8qo00130h Li-Jun Hu 1, 2, 3, 4 , Min-Jing Cheng 4, 5, 6, 7, 8 , Jia-Qing Cao 4, 5, 6, 7 , Li-Ping Zhong 4, 8, 9, 10 , Ya-Jian Hu 4, 8, 9, 10 , Ying Wang 4, 5, 6, 7 , Lei Wang 4, 5, 6, 7 , Wen-Cai Ye 1, 2, 3, 4, 5 , Chuang-Chuang Li 4, 8, 9, 10
Affiliation
|
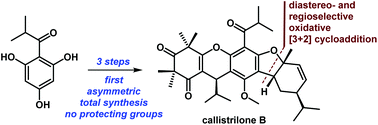
A very concise approach for the first asymmetric and scalable total syntheses of callistrilones B, G and J has been developed. These syntheses proceeded in only 3–4 steps from the commercially available (−)-α-phellandrene (8), without the need for protecting groups. The key feature of the syntheses was a biomimetic, diastereo- and regioselective oxidative [3 + 2] cycloaddition.
中文翻译:

Callistrilones B,G和J的不对称总合成†
已经开发出一种非常简洁的方法来用于Callistrilones B,G和J的第一个不对称和可扩展的总合成。从市售(-)-α-芹烯(8)进行这些合成仅需3-4步,而无需保护基团。合成的关键特征是仿生,非对映和区域选择性氧化[3 + 2]环加成反应。
更新日期:2018-03-15
中文翻译:

Callistrilones B,G和J的不对称总合成†
已经开发出一种非常简洁的方法来用于Callistrilones B,G和J的第一个不对称和可扩展的总合成。从市售(-)-α-芹烯(8)进行这些合成仅需3-4步,而无需保护基团。合成的关键特征是仿生,非对映和区域选择性氧化[3 + 2]环加成反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号