当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the mechanism of thermally driven thiol-Michael dynamic covalent chemistry†
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8ob00397a Borui Zhang 1, 2, 3, 4 , Progyateg Chakma 1, 2, 3, 4 , Max P. Shulman 1, 2, 3, 4 , Jun Ke 1, 2, 3, 4 , Zachary A. Digby 1, 2, 3, 4 , Dominik Konkolewicz 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8ob00397a Borui Zhang 1, 2, 3, 4 , Progyateg Chakma 1, 2, 3, 4 , Max P. Shulman 1, 2, 3, 4 , Jun Ke 1, 2, 3, 4 , Zachary A. Digby 1, 2, 3, 4 , Dominik Konkolewicz 1, 2, 3, 4
Affiliation
|
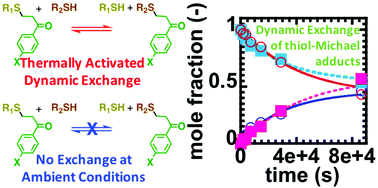
The kinetics and mechanism of the thermally activated dynamic covalent exchange of thiol-Michael adducts is investigated. A model system of thiol-Michael adducts between thiophenol and phenylvinylketone derivatives and adducts between 2-mercaptoethanol phenylvinylketone derivatives in N,N-dimethylformamide (DMF) at elevated temperatures is used to probe the underlying exchange mechanism. The kinetic data show negligible free Michael acceptor, which is consistent with the highly efficient thiol-Michael reaction being a “click”-like reaction that significantly favors the adduct form. At elevated temperatures of 90 °C in DMF the thiol-Michael adducts reach equilibrium after 24 h, although equilibration did not occur within 24 h at 60 °C or 75 °C, and negligible exchange occurs under ambient conditions. A kinetic model was developed to describe the dynamic covalent exchange and equilibration. The experimental and simulation kinetic data of dynamic covalent exchange are consistent with the thiol-Michael adducts undergoing a retro-Michael reaction, followed by subsequent addition of a free thiol to the liberated Michael acceptor. Kinetic analysis is consistent with the fragmentation, or retro-Michael reaction, being the rate-determining step in the dynamic covalent exchange. This suggests that the key step in dynamic covalent exchange is not enhanced by addition of free thiol or free Michael acceptor, since the addition reaction is much faster than the retro-Michael reaction. This fundamental study will guide the design of organic compounds, materials, and bioconjugates that utilize the thermally activated dynamic covalent thiol-Michael linkages.
中文翻译:

探索热驱动硫醇-迈克尔动态共价化学的机理†
研究了硫醇-迈克尔加合物的热活化动态共价交换的动力学和机理。N,N中硫酚与苯基乙烯基酮衍生物之间的硫醇-迈克尔加成物和2-巯基乙醇苯基乙烯基酮衍生物之间的加成物的模型体系。高温下的-二甲基甲酰胺(DMF)用于探测潜在的交换机制。动力学数据显示游离的迈克尔受体可忽略不计,这与高效硫醇-迈克尔反应是“点击”状反应非常一致,该反应明显有利于加合物形式。在DMF中90°C的高温下,硫醇-迈克尔加合物在24 h后达到平衡,尽管在60°C或75°C的24 h内未发生平衡,并且在环境条件下交换可忽略不计。建立了动力学模型来描述动态共价交换和平衡。动态共价交换的实验和模拟动力学数据与经历逆迈克尔反应的硫醇-迈克尔加合物一致,随后将游离硫醇添加到释放的迈克尔受体中。动力学分析与断裂或逆迈克尔反应一致,后者是动态共价交换中决定速率的步骤。这表明动态共价交换的关键步骤没有通过添加游离硫醇或游离迈克尔受体来增强,因为添加反应比逆迈克尔反应要快得多。这项基础研究将指导利用热活化动态共价硫醇-迈克尔键的有机化合物,材料和生物共轭物的设计。因为加成反应比逆迈克尔反应快得多。这项基础研究将指导利用热活化动态共价硫醇-迈克尔键的有机化合物,材料和生物共轭物的设计。因为加成反应比逆迈克尔反应快得多。这项基础研究将指导利用热活化动态共价硫醇-迈克尔键的有机化合物,材料和生物共轭物的设计。
更新日期:2018-03-15
中文翻译:

探索热驱动硫醇-迈克尔动态共价化学的机理†
研究了硫醇-迈克尔加合物的热活化动态共价交换的动力学和机理。N,N中硫酚与苯基乙烯基酮衍生物之间的硫醇-迈克尔加成物和2-巯基乙醇苯基乙烯基酮衍生物之间的加成物的模型体系。高温下的-二甲基甲酰胺(DMF)用于探测潜在的交换机制。动力学数据显示游离的迈克尔受体可忽略不计,这与高效硫醇-迈克尔反应是“点击”状反应非常一致,该反应明显有利于加合物形式。在DMF中90°C的高温下,硫醇-迈克尔加合物在24 h后达到平衡,尽管在60°C或75°C的24 h内未发生平衡,并且在环境条件下交换可忽略不计。建立了动力学模型来描述动态共价交换和平衡。动态共价交换的实验和模拟动力学数据与经历逆迈克尔反应的硫醇-迈克尔加合物一致,随后将游离硫醇添加到释放的迈克尔受体中。动力学分析与断裂或逆迈克尔反应一致,后者是动态共价交换中决定速率的步骤。这表明动态共价交换的关键步骤没有通过添加游离硫醇或游离迈克尔受体来增强,因为添加反应比逆迈克尔反应要快得多。这项基础研究将指导利用热活化动态共价硫醇-迈克尔键的有机化合物,材料和生物共轭物的设计。因为加成反应比逆迈克尔反应快得多。这项基础研究将指导利用热活化动态共价硫醇-迈克尔键的有机化合物,材料和生物共轭物的设计。因为加成反应比逆迈克尔反应快得多。这项基础研究将指导利用热活化动态共价硫醇-迈克尔键的有机化合物,材料和生物共轭物的设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号