当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single-particle measurements of electrochemical kinetics in NMC and NCA cathodes for Li-ion batteries†
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8ee00001h Ping-Chun Tsai 1, 1, 2, 3, 4 , Bohua Wen 1, 2, 3, 4 , Mark Wolfman 4, 5, 6, 7 , Min-Ju Choe 1, 4, 8, 9 , Menghsuan Sam Pan 1, 2, 3, 4 , Liang Su 1, 2, 3, 4 , Katsuyo Thornton 1, 4, 8, 9 , Jordi Cabana 4, 5, 6, 7 , Yet-Ming Chiang 1, 2, 3, 4
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8ee00001h Ping-Chun Tsai 1, 1, 2, 3, 4 , Bohua Wen 1, 2, 3, 4 , Mark Wolfman 4, 5, 6, 7 , Min-Ju Choe 1, 4, 8, 9 , Menghsuan Sam Pan 1, 2, 3, 4 , Liang Su 1, 2, 3, 4 , Katsuyo Thornton 1, 4, 8, 9 , Jordi Cabana 4, 5, 6, 7 , Yet-Ming Chiang 1, 2, 3, 4
Affiliation
|
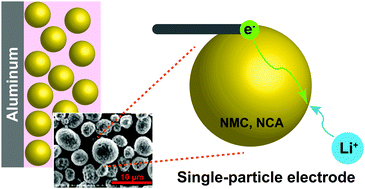
The electrochemical kinetics of battery electrodes at the single-particle scale are measured as a function of state-of-charge, and interpreted with the aid of concurrent transmission X-ray microscopy (TXM) of the evolving particle microstructure. An electrochemical cell operating with near-picoampere current resolution is used to characterize single secondary particles of two widely-used cathode compounds, NMC333 and NCA. Interfacial charge transfer kinetics are found to vary by two orders of magnitude with state-of-charge (SOC) in both materials, but the origin of the SOC dependence differs greatly. NCA behavior is dominated by electrochemically-induced microfracture, although thin binder coatings significantly ameliorate mechanical degradation, while NMC333 demonstrates strongly increasing interfacial reaction rates with SOC for chemical reasons. Micro-PITT is used to separate interfacial and bulk transport rates, and show that for commercially relevant particle sizes, interfacial transport is rate-limiting at low SOC, while mixed-control dominates at higher SOC. These results provide mechanistic insight into the mesoscale kinetics of ion intercalation compounds, which can guide the development of high performance rechargeable batteries.
中文翻译:

锂离子电池NMC和NCA阴极中电化学动力学的单粒子测量†
测量电池电极在单粒子尺度上的电化学动力学,它是荷电状态的函数,并借助不断演变的粒子微观结构的同时透射X射线显微镜(TXM)进行解释。使用以近皮安培电流分辨率工作的电化学电池来表征两种广泛使用的阴极化合物NMC333和NCA的单个次级粒子。在两种材料中,界面电荷转移动力学随电荷状态(SOC)的变化而变化了两个数量级,但是SOC依赖性的起源却有很大差异。NCA行为主要由电化学诱导的微裂纹控制,尽管薄的粘合剂涂层可显着改善机械降解,而NMC333由于化学原因显示出与SOC的界面反应速率大大提高。Micro-PITT用于分离界面传输速率和整体传输速率,并表明对于商业相关的粒径,界面传输在低SOC下是限速的,而混合控制在较高SOC下占主导。这些结果为离子嵌入化合物的介观动力学提供了机械学见识,可指导高性能可充电电池的开发。
更新日期:2018-03-15
中文翻译:

锂离子电池NMC和NCA阴极中电化学动力学的单粒子测量†
测量电池电极在单粒子尺度上的电化学动力学,它是荷电状态的函数,并借助不断演变的粒子微观结构的同时透射X射线显微镜(TXM)进行解释。使用以近皮安培电流分辨率工作的电化学电池来表征两种广泛使用的阴极化合物NMC333和NCA的单个次级粒子。在两种材料中,界面电荷转移动力学随电荷状态(SOC)的变化而变化了两个数量级,但是SOC依赖性的起源却有很大差异。NCA行为主要由电化学诱导的微裂纹控制,尽管薄的粘合剂涂层可显着改善机械降解,而NMC333由于化学原因显示出与SOC的界面反应速率大大提高。Micro-PITT用于分离界面传输速率和整体传输速率,并表明对于商业相关的粒径,界面传输在低SOC下是限速的,而混合控制在较高SOC下占主导。这些结果为离子嵌入化合物的介观动力学提供了机械学见识,可指导高性能可充电电池的开发。











































 京公网安备 11010802027423号
京公网安备 11010802027423号