当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Covalent and selective immobilization of GST fusion proteins with fluorophosphonate-based probes†
Chemical Communications ( IF 4.9 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c7cc08888d Xiafeng Wang 1, 2, 3, 4, 5 , Tianlin Guo 1, 2, 3, 4, 5 , Jiahui Chen 1, 2, 3, 4, 5 , Xiaofeng Li 1, 2, 3, 4, 5 , Yiqing Zhou 1, 2, 3, 4, 5 , Zhengying Pan 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c7cc08888d Xiafeng Wang 1, 2, 3, 4, 5 , Tianlin Guo 1, 2, 3, 4, 5 , Jiahui Chen 1, 2, 3, 4, 5 , Xiaofeng Li 1, 2, 3, 4, 5 , Yiqing Zhou 1, 2, 3, 4, 5 , Zhengying Pan 1, 2, 3, 4, 5
Affiliation
|
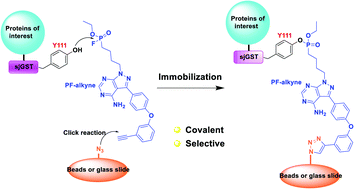
Using GST fusion protein tags is an attractive approach for protein immobilization. Here we report that pyrimidine-based small-molecule probes with a fluorophosphonate reactive group could specifically react with the tyrosine-111 residue of the Schistosoma japonicum GST (sjGST) tag, and these probes could rapidly and site-selectively immobilize sjGST fusion proteins while preserving their activities.
中文翻译:

GST融合蛋白与基于氟膦酸酯的探针共价和选择性固定†
使用GST融合蛋白标签是固定蛋白的一种有吸引力的方法。在这里我们报告具有氟膦酸酯反应性基团的基于嘧啶的小分子探针可以与日本血吸虫GST(sjGST)标签的酪氨酸111残基特异性反应,并且这些探针可以在保留保留的前提下快速和选择性地固定sjGST融合蛋白他们的活动。
更新日期:2018-03-15
中文翻译:

GST融合蛋白与基于氟膦酸酯的探针共价和选择性固定†
使用GST融合蛋白标签是固定蛋白的一种有吸引力的方法。在这里我们报告具有氟膦酸酯反应性基团的基于嘧啶的小分子探针可以与日本血吸虫GST(sjGST)标签的酪氨酸111残基特异性反应,并且这些探针可以在保留保留的前提下快速和选择性地固定sjGST融合蛋白他们的活动。



























 京公网安备 11010802027423号
京公网安备 11010802027423号