当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triflic acid-catalyzed metal-free synthesis of (E)-2-cyanoacrylamides and 3-substituted azetidine-2,4-diones†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c7nj05169g Bapurao D. Rupanwar 1, 2, 3, 4 , Santosh S. Chavan 1, 2, 3, 4 , Anil M. Shelke 1, 2, 3, 4 , Gurunath M. Suryavanshi 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c7nj05169g Bapurao D. Rupanwar 1, 2, 3, 4 , Santosh S. Chavan 1, 2, 3, 4 , Anil M. Shelke 1, 2, 3, 4 , Gurunath M. Suryavanshi 1, 2, 3, 4
Affiliation
|
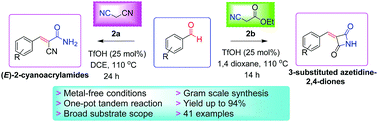
A TfOH-catalyzed highly efficient synthesis of biologically active (E)-2-cyanoacrylamides and 3-substituted azetidine-2,4-diones has been reported with 64–94% yields under metal-free conditions. The reaction proceeds through sequential Knoevenagel condensation/stereoselective in situ monohydration of nitrile or C–N cyclization protocol in one-pot. The attractive features of this tandem process are moderate reaction conditions, high atom economy, broad substrate scope, gram-scale reaction and ease of operation.
中文翻译:

三氟甲磺酸催化的无金属合成(E)-2-氰基丙烯酰胺和3-取代的氮杂环丁烷2,4-二酮†
据报道,在无金属条件下,TfOH催化可高效合成具有生物活性的(E)-2-氰基丙烯酰胺和3-取代的氮杂环丁烷2,4-二酮,收率为64-94%。反应通过一锅顺序进行的Knoevenagel缩合反应/腈选择性地原位单水合或CN环化方案进行。该串联方法的吸引人的特征是中等反应条件,高原子经济性,广泛的底物范围,克级反应和易于操作。
更新日期:2018-03-15
中文翻译:

三氟甲磺酸催化的无金属合成(E)-2-氰基丙烯酰胺和3-取代的氮杂环丁烷2,4-二酮†
据报道,在无金属条件下,TfOH催化可高效合成具有生物活性的(E)-2-氰基丙烯酰胺和3-取代的氮杂环丁烷2,4-二酮,收率为64-94%。反应通过一锅顺序进行的Knoevenagel缩合反应/腈选择性地原位单水合或CN环化方案进行。该串联方法的吸引人的特征是中等反应条件,高原子经济性,广泛的底物范围,克级反应和易于操作。











































 京公网安备 11010802027423号
京公网安备 11010802027423号