当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biochemical and biophysical insights into the metal binding spectrum and bioactivity of arginase of Entamoeba histolytica†
Metallomics ( IF 3.4 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8mt00002f Anjali Malik 1, 2, 3, 4 , Harvijay Singh 1, 2, 3, 4 , Akshay Pareek 1, 2, 3, 4 , Shailly Tomar 1, 2, 3, 4
Metallomics ( IF 3.4 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1039/c8mt00002f Anjali Malik 1, 2, 3, 4 , Harvijay Singh 1, 2, 3, 4 , Akshay Pareek 1, 2, 3, 4 , Shailly Tomar 1, 2, 3, 4
Affiliation
|
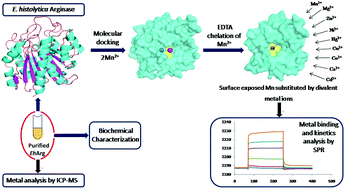
The human protozoan pathogens possess the essential metalloenzyme arginase (Arg) which catalyses the catabolism of L-arginine to L-ornithine and urea. This being the first committed step in polyamine biosynthesis is a potential drug target for protozoan diseases. In pathogenic organisms, arginase plays a crucial role in depleting host L-arginine, a substrate for nitric oxide synthase (NOS) that participates in protective immunity, thereby evading host immune response. In this study, the metal binding spectrum of EhArg has been determined. This study focuses on the biochemical and biophysical characterization of arginase from Entamoeba histolytica (EhArg), majorly characterizing the bivalent metal selectivity and metal binding kinetics of purified EhArg using Surface Plasmon Resonance and inductively coupled plasma mass spectroscopy. Investigation of the active site chemistry and total metal content using molecular docking and ICP-MS unraveled the fact that two Mn2+ ions are required for the enzyme to be fully functional. However, chelating loosely bound Mn2+ and replacing it with a variety of bivalent metal ions including Mg2+, Zn2+, Ni2+, Hg2+, Cu2+, Co2+, Ca2+ and Cd2+ retains its enzymatic activity. Further, the role of nine bivalent ions in the activation of EhArg was studied thermodynamically and biochemically. Phylogenetic and sequence analysis and oligomerization studies of EhArg show that unlike other eukaryotic arginases, EhArg exists in monomeric and dimeric form in solution and shows the highest similarity with bacterial arginase. This study unveiled interesting facts about EhArg that the enzyme has evolved to utilize available metal ion cofactors and survive the inhospitable environment within the host.
中文翻译:

生化和生物物理洞察力对溶组织变形杆菌的金属结合光谱和精氨酸酶的生物活性†
人类原生动物病原体具有基本金属酶精氨酸(精氨酸),其催化的分解代谢大号精氨酸到大号鸟氨酸和尿素。这是多胺生物合成的第一步,是原生动物疾病的潜在药物靶标。在病原生物中,精氨酸酶在消耗宿主L-精氨酸中起着至关重要的作用,L-精氨酸是一氧化氮合酶(NOS)的底物,参与保护性免疫,从而逃避了宿主的免疫反应。在这项研究中,已确定了EhArg的金属结合光谱。这项研究侧重于组织解脂变形杆菌的精氨酸酶的生化和生物物理表征(EhArg),主要使用表面等离振子共振和电感耦合等离子体质谱法表征纯化的EhArg的二价金属选择性和金属结合动力学。使用分子对接和ICP-MS进行的活性位化学和总金属含量的研究揭示了这样的事实,即该酶要完全发挥功能需要两个Mn 2+离子。然而,螯合松散结合的Mn 2+并用各种二价金属离子替代,包括Mg 2 +,Zn 2 +,Ni 2 +,Hg 2 +,Cu 2 +,Co 2 +,Ca 2+和Cd 2+保留其酶活性。此外,热力学和生物化学研究了九个二价离子在EhArg活化中的作用。EhArg的系统发生,序列分析和寡聚研究表明,与其他真核精氨酸酶不同,EhArg以单体和二聚体形式存在于溶液中,与细菌精氨酸酶的相似性最高。这项研究揭示了有关EhArg的有趣事实,即该酶已进化为利用可利用的金属离子辅因子,并在宿主内恶劣的环境中生存。
更新日期:2018-03-15
中文翻译:

生化和生物物理洞察力对溶组织变形杆菌的金属结合光谱和精氨酸酶的生物活性†
人类原生动物病原体具有基本金属酶精氨酸(精氨酸),其催化的分解代谢大号精氨酸到大号鸟氨酸和尿素。这是多胺生物合成的第一步,是原生动物疾病的潜在药物靶标。在病原生物中,精氨酸酶在消耗宿主L-精氨酸中起着至关重要的作用,L-精氨酸是一氧化氮合酶(NOS)的底物,参与保护性免疫,从而逃避了宿主的免疫反应。在这项研究中,已确定了EhArg的金属结合光谱。这项研究侧重于组织解脂变形杆菌的精氨酸酶的生化和生物物理表征(EhArg),主要使用表面等离振子共振和电感耦合等离子体质谱法表征纯化的EhArg的二价金属选择性和金属结合动力学。使用分子对接和ICP-MS进行的活性位化学和总金属含量的研究揭示了这样的事实,即该酶要完全发挥功能需要两个Mn 2+离子。然而,螯合松散结合的Mn 2+并用各种二价金属离子替代,包括Mg 2 +,Zn 2 +,Ni 2 +,Hg 2 +,Cu 2 +,Co 2 +,Ca 2+和Cd 2+保留其酶活性。此外,热力学和生物化学研究了九个二价离子在EhArg活化中的作用。EhArg的系统发生,序列分析和寡聚研究表明,与其他真核精氨酸酶不同,EhArg以单体和二聚体形式存在于溶液中,与细菌精氨酸酶的相似性最高。这项研究揭示了有关EhArg的有趣事实,即该酶已进化为利用可利用的金属离子辅因子,并在宿主内恶劣的环境中生存。



























 京公网安备 11010802027423号
京公网安备 11010802027423号