当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimization of a β-Lactam Scaffold for Antibacterial Activity via the Inhibition of Bacterial Type I Signal Peptidase
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00064 Chien-Hung Yeh 1 , Shawn I. Walsh 1 , Arryn Craney 1 , M. Greg Tabor 1 , Ana-Florina Voica 1 , Ramkrishna Adhikary 1 , Sydney E. Morris 1 , Floyd E. Romesberg 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-03-15 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00064 Chien-Hung Yeh 1 , Shawn I. Walsh 1 , Arryn Craney 1 , M. Greg Tabor 1 , Ana-Florina Voica 1 , Ramkrishna Adhikary 1 , Sydney E. Morris 1 , Floyd E. Romesberg 1
Affiliation
|
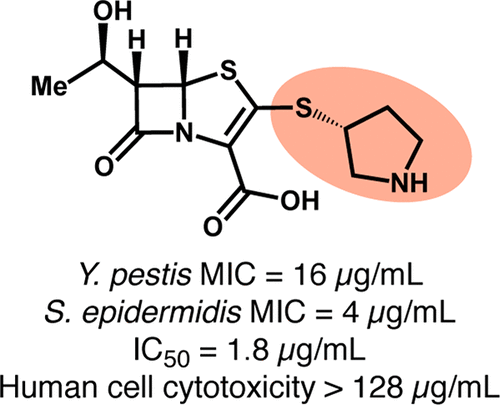
β-Lactam antibiotics, one of the most important class of human therapeutics, act via the inhibition of penicillin-binding proteins (PBPs). The unparalleled success in their development has inspired efforts to develop them as inhibitors of other targets. Bacterial type I signal peptidase is evolutionarily related to the PBPs, but the stereochemistry of its substrates and its catalytic mechanism suggest that β-lactams with the 5S stereochemistry, as opposed to the 5R stereochemistry of the traditional β-lactams, would be required for inhibition. We report the synthesis and evaluation of a variety of 5S penem derivatives and identify several with promising activity against both a Gram-positive and a Gram-negative bacterial pathogen. To our knowledge these are the first 5S β-lactams to possess significant antibacterial activity and the first β-lactams imparted with antibacterial activity via optimization of the inhibition of a target other than a PBP. Along with the privileged status of their scaffold and the promise of bacterial signal peptidase I (SPase) as a target, this activity makes these compounds promising leads for development as novel therapeutics.
中文翻译:

通过抑制细菌I型信号肽酶优化β-内酰胺支架的抗菌活性
β-内酰胺抗生素是人类治疗药物中最重要的一类,它通过抑制青霉素结合蛋白(PBPs)发挥作用。他们的发展无与伦比的成功激发了人们努力将其发展为其他靶标的抑制剂。细菌I型信号肽酶与PBPs进化相关,但是其底物的立体化学及其催化机理表明,与传统的β-内酰胺类的5 R立体化学相反,具有5 S立体化学的β-内酰胺是必需的。抑制。我们报告了各种5 S的综合和评估Penem衍生物,并鉴定出对革兰氏阳性和革兰氏阴性细菌病原体都具有前景的活性。据我们所知,这是第5个小号β内酰胺拥有显著的抗菌活性和第一β内酰胺类抗生素具有抗菌活性比通过一个PBP其他目标的抑制优化传授。连同其支架的优越地位以及以细菌信号肽酶I(SPase)为靶标的希望,这种活性使这些化合物有望成为开发新型治疗剂的线索。
更新日期:2018-03-15
中文翻译:

通过抑制细菌I型信号肽酶优化β-内酰胺支架的抗菌活性
β-内酰胺抗生素是人类治疗药物中最重要的一类,它通过抑制青霉素结合蛋白(PBPs)发挥作用。他们的发展无与伦比的成功激发了人们努力将其发展为其他靶标的抑制剂。细菌I型信号肽酶与PBPs进化相关,但是其底物的立体化学及其催化机理表明,与传统的β-内酰胺类的5 R立体化学相反,具有5 S立体化学的β-内酰胺是必需的。抑制。我们报告了各种5 S的综合和评估Penem衍生物,并鉴定出对革兰氏阳性和革兰氏阴性细菌病原体都具有前景的活性。据我们所知,这是第5个小号β内酰胺拥有显著的抗菌活性和第一β内酰胺类抗生素具有抗菌活性比通过一个PBP其他目标的抑制优化传授。连同其支架的优越地位以及以细菌信号肽酶I(SPase)为靶标的希望,这种活性使这些化合物有望成为开发新型治疗剂的线索。











































 京公网安备 11010802027423号
京公网安备 11010802027423号