当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Efficient Method for Determining the Relative Configuration of Furofuran Lignans by 1H NMR Spectroscopy
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jnatprod.8b00044 Si-Yuan Shao 1 , Ya-Nan Yang 1 , Zi-Ming Feng 1 , Jian-Shuang Jiang 1 , Pei-Cheng Zhang 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jnatprod.8b00044 Si-Yuan Shao 1 , Ya-Nan Yang 1 , Zi-Ming Feng 1 , Jian-Shuang Jiang 1 , Pei-Cheng Zhang 1
Affiliation
|
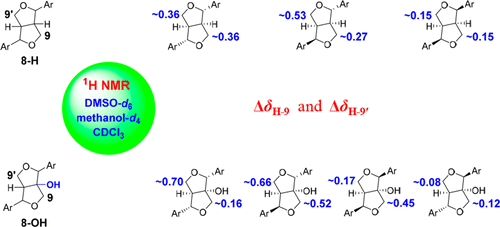
An efficient 1H NMR spectroscopic approach for determining the relative configurations of lignans with a 7,9′:7′,9-diepoxy moiety has been established. Using the chemical shift differences of H2-9 and H2-9′ (ΔδH-9 and ΔδH-9′), the configurations of 8-H and 8-OH furofuran lignans can be rapidly and conveniently determined. The rule is applicable for data acquired in DMSO-d6, methanol-d4, or CDCl3. Notably, the rule should be applied carefully when the C-2 or C-6 substituent of the aromatic rings may alter the dominant conformers of the furofuran moiety.
中文翻译:

1 H NMR光谱法测定呋喃呋喃木质素相对构型的有效方法
已经建立了用于确定具有7,9':7',9-二乙氧基部分的木脂素的相对构型的有效的1 H NMR光谱方法。利用H 2 -9和H 2 -9'的化学位移差(ΔδH -9和ΔδH -9'),可以快速方便地确定8-H和8-OH呋喃呋喃木脂素的构型。该规则适用于以DMSO- d 6,甲醇-d 4或CDCl 3采集的数据。值得注意的是,当芳环的C-2或C-6取代基可能改变呋喃呋喃部分的主要构象时,应谨慎应用该规则。
更新日期:2018-03-14
中文翻译:

1 H NMR光谱法测定呋喃呋喃木质素相对构型的有效方法
已经建立了用于确定具有7,9':7',9-二乙氧基部分的木脂素的相对构型的有效的1 H NMR光谱方法。利用H 2 -9和H 2 -9'的化学位移差(ΔδH -9和ΔδH -9'),可以快速方便地确定8-H和8-OH呋喃呋喃木脂素的构型。该规则适用于以DMSO- d 6,甲醇-d 4或CDCl 3采集的数据。值得注意的是,当芳环的C-2或C-6取代基可能改变呋喃呋喃部分的主要构象时,应谨慎应用该规则。











































 京公网安备 11010802027423号
京公网安备 11010802027423号