当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Using stereoretention for the synthesis of E-macrocycles with ruthenium-based olefin metathesis catalysts†
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c8sc00435h Tonia S. Ahmed 1, 2, 3, 4, 5 , T. Patrick Montgomery 1, 2, 3, 4, 5 , Robert H. Grubbs 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c8sc00435h Tonia S. Ahmed 1, 2, 3, 4, 5 , T. Patrick Montgomery 1, 2, 3, 4, 5 , Robert H. Grubbs 1, 2, 3, 4, 5
Affiliation
|
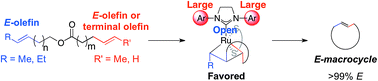
The synthesis of E-macrocycles is achieved using stereoretentive, Ru-based olefin metathesis catalysts supported by dithiolate ligands. Kinetic studies elucidate marked differences in activity among the catalysts tested, with catalyst 4 providing meaningful yields of products in much shorter reaction times than stereoretentive catalysts 2 and 3. Macrocycles were generated with excellent selectivity (>99% E) and in moderate to high yields (47–80% yield) from diene starting materials bearing two E-configured olefins. A variety of rings were constructed, ranging from 12- to 18-membered macrocycles, including the antibiotic recifeiolide.
中文翻译:

使用立构保留与钌基烯烃复分解催化剂合成E-大环†
E-大环的合成是使用二硫醇盐配体负载的立体保持性,Ru基烯烃复分解催化剂来实现的。动力学研究阐明了所测试的催化剂之间活性的显着差异,其中催化剂4在比立体保持催化剂2和3短得多的反应时间内提供了有意义的产物收率。大环以优异的选择性(> 99%E)和中等至高收率(47-80%收率)从带有两种E-构型烯烃的二烯原料中生成。构建了各种环,范围从12到18元的大环化合物,包括抗生素回收苯环内酯。
更新日期:2018-03-14
中文翻译:

使用立构保留与钌基烯烃复分解催化剂合成E-大环†
E-大环的合成是使用二硫醇盐配体负载的立体保持性,Ru基烯烃复分解催化剂来实现的。动力学研究阐明了所测试的催化剂之间活性的显着差异,其中催化剂4在比立体保持催化剂2和3短得多的反应时间内提供了有意义的产物收率。大环以优异的选择性(> 99%E)和中等至高收率(47-80%收率)从带有两种E-构型烯烃的二烯原料中生成。构建了各种环,范围从12到18元的大环化合物,包括抗生素回收苯环内酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号