Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-03-14 , DOI: 10.1016/j.bmcl.2018.03.027 Takumi Ohara , Masato Kaneda , Tomo Saito , Nobutaka Fujii , Hiroaki Ohno , Shinya Oishi
|
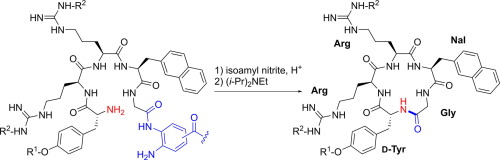
A head-to-tail macrocyclization protocol for the preparation of cysteine-free cyclic peptides was investigated. The o-aminoanilide linker constructed in the peptide sequence by a standard Fmoc-based peptide synthesis procedure was subjected to nitrite-mediated activation under acidic conditions toward N-acyl benzotriazole as the active ester species. The subsequent cyclization smoothly proceeded by neutralization in the presence of additives such as 1-hydroxybenzotriazole (HOBt) and 1-hydroxy-7-azabenzotriazole (HOAt) to afford the expected cyclic pentapeptide, a CXCR4 antagonist. The cyclization efficiencies were dependent on the precursor open-chain sequence. The application of this step-wise activation-cyclization protocol to microflow reaction systems is also described.
中文翻译:

使用邻氨基苯胺接头从头到尾对无半胱氨酸的肽进行大环化
研究了从头到尾的大环化方案,用于制备无半胱氨酸的环肽。通过基于标准Fmoc的肽合成方法在肽序列中构建的邻氨基苯胺基接头在酸性条件下经受亚硝酸盐介导的活化,以活化为N-酰基苯并三唑。随后的环化反应通过在存在添加剂(例如1-羟基苯并三唑(HOBt)和1-羟基-7-氮杂苯并三唑(HOAt))的存在下进行中和而顺利进行,以提供预期的环状五肽(一种CXCR4拮抗剂)。环化效率取决于前体开链序列。还描述了该逐步活化环化方案在微流反应系统中的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号