Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Csp3–H Bond mono-Aroyloxylation of O-Alkyl Substituted 2,4,6-Trimethoxybenzaldoxime Ethers
Synlett ( IF 1.7 ) Pub Date : 2018-03-14 , DOI: 10.1055/s-0036-1609346 Wen-Li Qiao 1 , Ling-Yan Shao 1 , Ya-Hua Hu 1 , Li-Hao Xing 1 , Ke-Zuan Deng 1 , Hong-Wei Liu 1 , Dao-Hua Liao 1 , Ya-Fei Ji 1
Synlett ( IF 1.7 ) Pub Date : 2018-03-14 , DOI: 10.1055/s-0036-1609346 Wen-Li Qiao 1 , Ling-Yan Shao 1 , Ya-Hua Hu 1 , Li-Hao Xing 1 , Ke-Zuan Deng 1 , Hong-Wei Liu 1 , Dao-Hua Liao 1 , Ya-Fei Ji 1
Affiliation
|
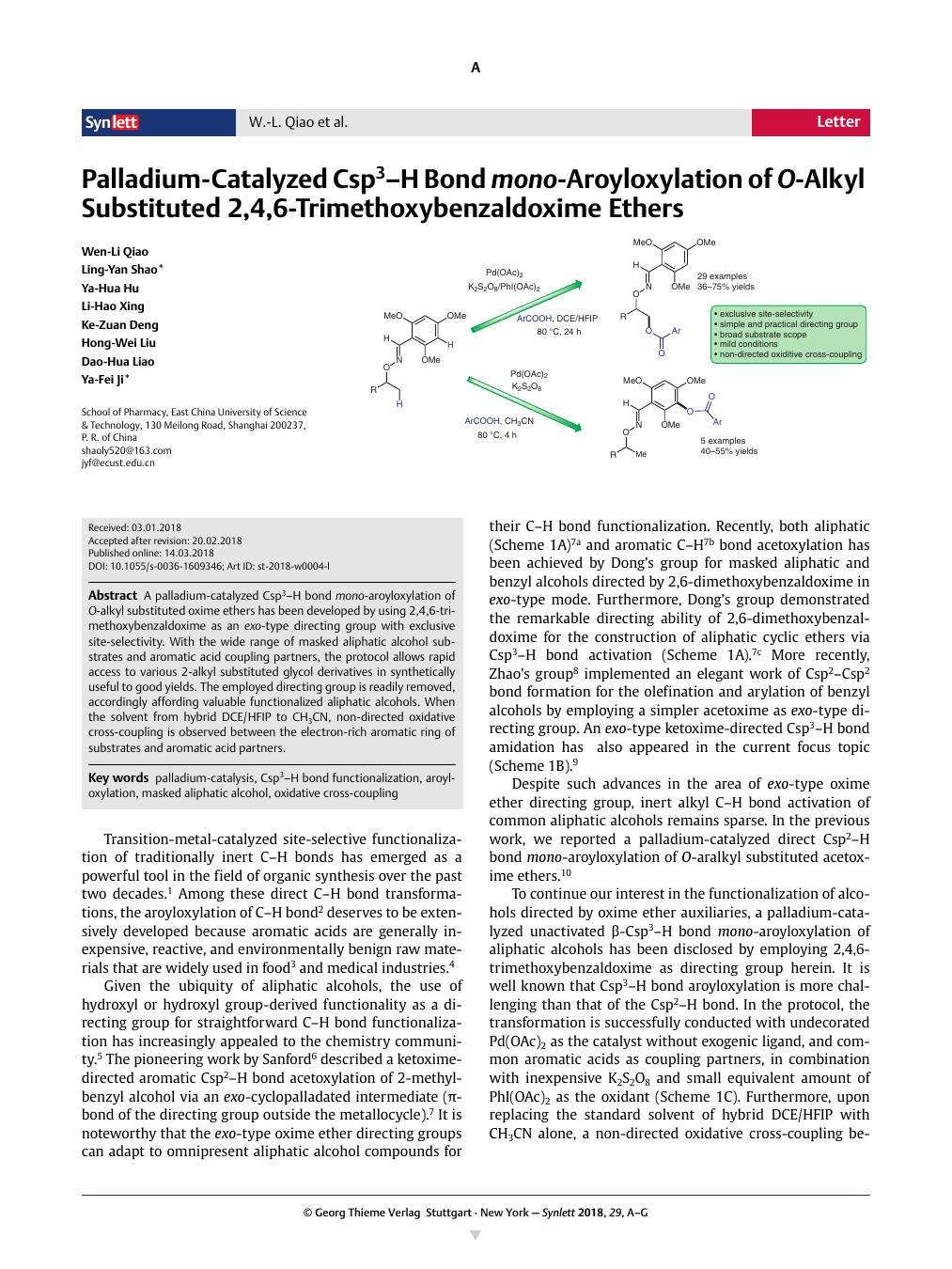
A palladium-catalyzed Csp3–H bond mono-aroyloxylation of O-alkyl substituted oxime ethers has been developed by using 2,4,6-trimethoxybenzaldoxime as an exo-type directing group with exclusive site-selectivity. With the wide range of masked aliphatic alcohol substrates and aromatic acid coupling partners, the protocol allows rapid access to various 2-alkyl substituted glycol derivatives in synthetically useful to good yields. The employed directing group is readily removed, accordingly affording valuable functionalized aliphatic alcohols. When the solvent from hybrid DCE/HFIP to CH3CN, non-directed oxidative cross-coupling is observed between the electron-rich aromatic ring of substrates and aromatic acid partners.
中文翻译:

O-烷基取代的 2,4,6-三甲氧基苯甲醛肟醚的钯催化 Csp3-H 键单芳酰氧基化
通过使用 2,4,6-三甲氧基苯甲醛肟作为具有独特位点选择性的外型导向基团,开发了钯催化的 Csp3-H 键单芳酰氧基化 O-烷基取代的肟醚。由于具有广泛的掩蔽脂肪醇底物和芳香酸偶联伙伴,该协议允许快速访问各种 2-烷基取代的乙二醇衍生物,以合成有用的高产率。使用的导向基团很容易去除,因此提供有价值的官能化脂肪醇。当溶剂从混合 DCE/HFIP 到 CH3CN 时,在底物的富电子芳环和芳酸伙伴之间观察到非定向氧化交叉偶联。
更新日期:2018-03-14
中文翻译:

O-烷基取代的 2,4,6-三甲氧基苯甲醛肟醚的钯催化 Csp3-H 键单芳酰氧基化
通过使用 2,4,6-三甲氧基苯甲醛肟作为具有独特位点选择性的外型导向基团,开发了钯催化的 Csp3-H 键单芳酰氧基化 O-烷基取代的肟醚。由于具有广泛的掩蔽脂肪醇底物和芳香酸偶联伙伴,该协议允许快速访问各种 2-烷基取代的乙二醇衍生物,以合成有用的高产率。使用的导向基团很容易去除,因此提供有价值的官能化脂肪醇。当溶剂从混合 DCE/HFIP 到 CH3CN 时,在底物的富电子芳环和芳酸伙伴之间观察到非定向氧化交叉偶联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号