当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A chiral Brønsted acid-catalyzed highly enantioselective Mannich-type reaction of α-diazo esters with in situ generated N-acyl ketimines†‡
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c8cc01436a Rajshekhar A. Unhale 1, 2, 3, 4 , Milon M. Sadhu 1, 2, 3, 4 , Sumit K. Ray 1, 2, 3, 4 , Rayhan G. Biswas 1, 2, 3, 4 , Vinod K. Singh 1, 1, 2, 3, 4
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c8cc01436a Rajshekhar A. Unhale 1, 2, 3, 4 , Milon M. Sadhu 1, 2, 3, 4 , Sumit K. Ray 1, 2, 3, 4 , Rayhan G. Biswas 1, 2, 3, 4 , Vinod K. Singh 1, 1, 2, 3, 4
Affiliation
|
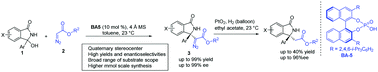
A chiral phosphoric acid-catalyzed asymmetric Mannich-type reaction of α-diazo esters with in situ generated N-acyl ketimines, derived from 3-hydroxyisoindolinones has been demonstrated in this communication. A variety of isoindolinone-based α-amino diazo esters bearing a quaternary stereogenic center were afforded in high yields (up to 99%) with excellent enantioselectivities (up to 99% ee). Furthermore, the synthetic utility of the products has been depicted by the hydrogenation of the diazo moiety of adducts.
中文翻译:

手性布朗斯台德酸催化α-重氮酯与原位生成的N-酰基酮亚胺的高对映选择性曼尼希型反应† ‡
在该文献中已经证明了手性磷酸催化α-重氮酯与原位生成的由3-羟基异吲哚满酮衍生的N-酰基酮亚胺的不对称曼尼希型反应。以高收率(高达99%)和优异的对映选择性(高达99%ee)提供了多种带有季立构中心的基于异吲哚啉酮的α-氨基重氮酯。此外,通过加合物的重氮部分的氢化描述了产物的合成效用。
更新日期:2018-03-14
中文翻译:

手性布朗斯台德酸催化α-重氮酯与原位生成的N-酰基酮亚胺的高对映选择性曼尼希型反应† ‡
在该文献中已经证明了手性磷酸催化α-重氮酯与原位生成的由3-羟基异吲哚满酮衍生的N-酰基酮亚胺的不对称曼尼希型反应。以高收率(高达99%)和优异的对映选择性(高达99%ee)提供了多种带有季立构中心的基于异吲哚啉酮的α-氨基重氮酯。此外,通过加合物的重氮部分的氢化描述了产物的合成效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号