当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A rhodium-catalyzed transannulation of N-(per)fluoroalkyl-1,2,3-triazoles under microwave conditions – a general route to N-(per)fluoroalkyl-substituted five-membered heterocycles†
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c8cc01446a Vladimir Motornov 1, 2, 3, 4, 5 , Athanasios Markos 1, 2, 3, 6, 7 , Petr Beier 1, 2, 3
Chemical Communications ( IF 4.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c8cc01446a Vladimir Motornov 1, 2, 3, 4, 5 , Athanasios Markos 1, 2, 3, 6, 7 , Petr Beier 1, 2, 3
Affiliation
|
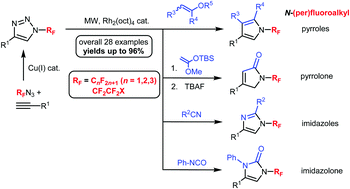
A rhodium-catalyzed transannulation via ring-opening of N-(per)fluoroalkyl-substituted 1,2,3-triazoles followed by cycloaddition with different nitriles, enol ethers, isocyanates and silyl ketene acetals under microwave heating provided a highly efficient route to previously unreported N-(per)fluoroalkyl-substituted imidazoles, pyrroles, imidazolones and pyrrolones, respectively. These reactions were found to be applicable to the synthesis of a variety of 5-membered heterocycles bearing different (per)fluoroalkyl substituents as well as both electron-donating and electron-withdrawing groups attached to the heterocyclic core.
中文翻译:

在微波条件下 铑催化的N-(全)氟烷基-1,2,3-三唑的环转移反应-N-(全)氟烷基取代的五元杂环的一般路线†
通过N-(全)氟烷基取代的1,2,3-三唑的开环,然后与不同的腈,烯醇醚,异氰酸酯和甲硅烷基烯酮缩醛进行环加成反应,铑催化的环转移反应提供了一种高效的方法未报道的N-(全)氟烷基取代的咪唑,吡咯,咪唑酮和吡咯烷酮。发现这些反应可用于合成带有不同(全)氟烷基取代基以及连接到杂环核上的给电子和吸电子基团的各种5元杂环。
更新日期:2018-03-14
中文翻译:

在微波条件下 铑催化的N-(全)氟烷基-1,2,3-三唑的环转移反应-N-(全)氟烷基取代的五元杂环的一般路线†
通过N-(全)氟烷基取代的1,2,3-三唑的开环,然后与不同的腈,烯醇醚,异氰酸酯和甲硅烷基烯酮缩醛进行环加成反应,铑催化的环转移反应提供了一种高效的方法未报道的N-(全)氟烷基取代的咪唑,吡咯,咪唑酮和吡咯烷酮。发现这些反应可用于合成带有不同(全)氟烷基取代基以及连接到杂环核上的给电子和吸电子基团的各种5元杂环。











































 京公网安备 11010802027423号
京公网安备 11010802027423号