当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Investigation of the Dopamine-2 Receptor Agonist Bromocriptine Binding to Dimeric D2HighR and D2LowR States
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jcim.7b00722 Ramin Ekhteiari Salmas 1 , Philip Seeman 2 , Matthias Stein 3 , Serdar Durdagi 1, 4
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jcim.7b00722 Ramin Ekhteiari Salmas 1 , Philip Seeman 2 , Matthias Stein 3 , Serdar Durdagi 1, 4
Affiliation
|
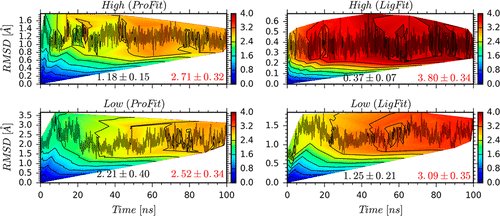
The active (D2HighR) and inactive (D2LowR) states of dimeric dopamine D2 receptor (D2R) models were investigated to clarify the binding mechanisms of the dopamine agonist bromocriptine, using Molecular Dynamics (MD) simulation. The aim of this comprehensive study was to investigate the critical effects of bromocriptine binding on each distinct receptor conformation. The different binding modes of the bromocriptine ligand in the active and inactive states have a significant effect on the conformational changes of the receptor. Based on the MM/GBSA approach, the calculated binding enthalpies of bromocriptine demonstrated selectivity toward the D2HighR active state. There is good agreement between the calculated and experimentally measured D2HighR selectivity. In the ligand-binding site, the key amino acids identified for D2HighR were Asp114(3.32) and Glu95(2.65), and for D2LowR, it was Ser193(5.42). Moreover, analysis of replicate MD trajectories demonstrated that the bromocriptine structure was more rigid at the D2HighR state and more flexible at the D2LowR state. However, the side chains of the ligand–receptor complex of D2HighR showed larger variations relative to the corresponding regions of D2LowR. The present study is part of an ongoing research program to study D2R conformational changes during ligand activation and to evaluate the conformational state selectivity for ligand binding.
中文翻译:

多巴胺2受体激动剂溴隐亭与二聚体D2高R和D2低R态结合的结构研究
使用分子动力学(MD)模拟研究了二聚多巴胺D2受体(D2R)模型的活跃(D2高R)和非活跃(D2低R)状态,以阐明多巴胺激动剂溴隐亭的结合机理。这项全面研究的目的是研究溴隐亭结合对每种不同受体构象的关键作用。处于活动状态和非活动状态的溴隐亭配体的不同结合模式对受体的构象变化具有重要影响。基于MM / GBSA方法,计算得出的溴隐亭的结合焓显示出对D2高R活性状态的选择性。还有就是计算和实验测量D2之间良好的一致性高R选择性。在配体结合位点,D2高R鉴定的关键氨基酸是Asp114(3.32)和Glu95(2.65),D2低R鉴定的关键氨基酸是Ser193(5.42)。此外,对重复MD轨迹的分析表明,溴隐亭的结构在D2高R状态下更刚性,而在D2低R状态下更灵活。但是,D2 High R的配体-受体复合物的侧链相对于D2 Low R的相应区域表现出较大的变化。本研究是正在进行的研究计划的一部分,该计划研究配体活化过程中D2R的构象变化并评估D2R的构象。配体结合的构象状态选择性。
更新日期:2018-03-14
中文翻译:

多巴胺2受体激动剂溴隐亭与二聚体D2高R和D2低R态结合的结构研究
使用分子动力学(MD)模拟研究了二聚多巴胺D2受体(D2R)模型的活跃(D2高R)和非活跃(D2低R)状态,以阐明多巴胺激动剂溴隐亭的结合机理。这项全面研究的目的是研究溴隐亭结合对每种不同受体构象的关键作用。处于活动状态和非活动状态的溴隐亭配体的不同结合模式对受体的构象变化具有重要影响。基于MM / GBSA方法,计算得出的溴隐亭的结合焓显示出对D2高R活性状态的选择性。还有就是计算和实验测量D2之间良好的一致性高R选择性。在配体结合位点,D2高R鉴定的关键氨基酸是Asp114(3.32)和Glu95(2.65),D2低R鉴定的关键氨基酸是Ser193(5.42)。此外,对重复MD轨迹的分析表明,溴隐亭的结构在D2高R状态下更刚性,而在D2低R状态下更灵活。但是,D2 High R的配体-受体复合物的侧链相对于D2 Low R的相应区域表现出较大的变化。本研究是正在进行的研究计划的一部分,该计划研究配体活化过程中D2R的构象变化并评估D2R的构象。配体结合的构象状态选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号