当前位置:
X-MOL 学术
›
Biomacromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Designing Highly Thermostable Lysozyme–Copolymer Conjugates: Focus on Effect of Polymer Concentration
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.biomac.8b00027 Chandan Kumar Choudhury 1 , Sidong Tu 1 , Igor Luzinov 1 , Sergiy Minko 2 , Olga Kuksenok 1
Biomacromolecules ( IF 5.5 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.biomac.8b00027 Chandan Kumar Choudhury 1 , Sidong Tu 1 , Igor Luzinov 1 , Sergiy Minko 2 , Olga Kuksenok 1
Affiliation
|
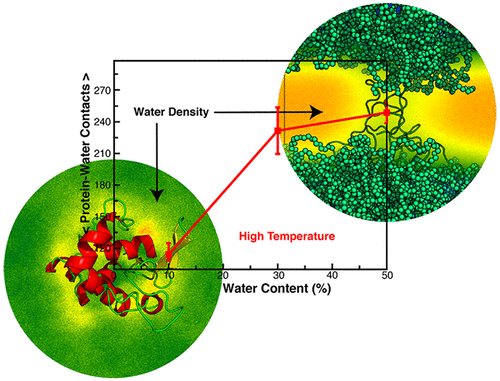
Designing biomaterials capable of functioning in harsh environments is vital for a range of applications. Using molecular dynamics simulations, we show that conjugating lysozymes with a copolymer [poly(GMA-stat-OEGMA)] comprising glycidyl methacrylate (GMA) and oligo(ethylene glycol) methyl ether methacrylate (OEGMA) results in a dramatic increase of stability of these enzymes at high temperatures provided that the concentration of the copolymer in the close vicinity of the enzyme exceeds a critical value. In our simulations, we use triads containing the same ratio of GMA to OEGMA units as in our recent experiments (N. S. Yadavalli et al., ACS Catalysis, 2017, 7, 8675). We focus on the dynamics of the conjugate at high temperatures and on its structural stability as a function of the copolymer/water content in the vicinity of the enzyme. We show that the dynamics of phase separation in the water–copolymer mixture surrounding the enzyme is critical for the structural stability of the enzyme. Specifically, restricting water access promotes the structural stability of the lysozyme at high temperatures. We identified critical water concentration below which we observe a robust stabilization; the phase separation is no longer observed at this low fraction of water so that the water domains promoting unfolding are no longer formed in the vicinity of the enzyme. This understanding provides a basis for future studies on designing a range of enzyme–copolymer conjugates with improved stability.
中文翻译:

设计高度耐热的溶菌酶-共聚物共轭物:关注聚合物浓度的影响
设计能够在恶劣环境下工作的生物材料对于一系列应用至关重要。使用分子动力学模拟,我们显示了溶菌酶与包含甲基丙烯酸缩水甘油酯(GMA)和低聚乙二醇乙二醇甲基丙烯酸甲酯(OEGMA)的共聚物[poly(GMA- stat -OEGMA)]结合的结果。前提条件是在酶附近的共聚物浓度超过临界值。在我们的模拟中,我们使用含有GMA的相同比率以OEGMA单元三单元组如在我们最近的实验(NS Yadavalli等人,ACS催化,2017年,7,8675)。我们关注于高温下缀合物的动力学及其作为酶附近共聚物/水含量的函数的结构稳定性。我们表明,酶周围的水-共聚物混合物中相分离的动力学对于酶的结构稳定性至关重要。具体地,限制水的进入促进了溶菌酶在高温下的结构稳定性。我们确定了临界水浓度,低于该临界水浓度,我们观察到了稳定的稳定性;在如此低的水含量下不再观察到相分离,因此促进展开的水域不再在酶附近形成。这种理解为将来设计一系列稳定性更高的酶-共聚物共轭物提供了基础。
更新日期:2018-03-14
中文翻译:

设计高度耐热的溶菌酶-共聚物共轭物:关注聚合物浓度的影响
设计能够在恶劣环境下工作的生物材料对于一系列应用至关重要。使用分子动力学模拟,我们显示了溶菌酶与包含甲基丙烯酸缩水甘油酯(GMA)和低聚乙二醇乙二醇甲基丙烯酸甲酯(OEGMA)的共聚物[poly(GMA- stat -OEGMA)]结合的结果。前提条件是在酶附近的共聚物浓度超过临界值。在我们的模拟中,我们使用含有GMA的相同比率以OEGMA单元三单元组如在我们最近的实验(NS Yadavalli等人,ACS催化,2017年,7,8675)。我们关注于高温下缀合物的动力学及其作为酶附近共聚物/水含量的函数的结构稳定性。我们表明,酶周围的水-共聚物混合物中相分离的动力学对于酶的结构稳定性至关重要。具体地,限制水的进入促进了溶菌酶在高温下的结构稳定性。我们确定了临界水浓度,低于该临界水浓度,我们观察到了稳定的稳定性;在如此低的水含量下不再观察到相分离,因此促进展开的水域不再在酶附近形成。这种理解为将来设计一系列稳定性更高的酶-共聚物共轭物提供了基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号