当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Effect of Piperine Pro-Nano Lipospheres on Direct Intestinal Phase II Metabolism: The Raloxifene Paradigm of Enhanced Oral Bioavailability
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b01090 Dvora Izgelov 1 , Irina Cherniakov 1 , Gefen Aldouby Bier 1 , Abraham J. Domb 1 , Amnon Hoffman 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b01090 Dvora Izgelov 1 , Irina Cherniakov 1 , Gefen Aldouby Bier 1 , Abraham J. Domb 1 , Amnon Hoffman 1
Affiliation
|
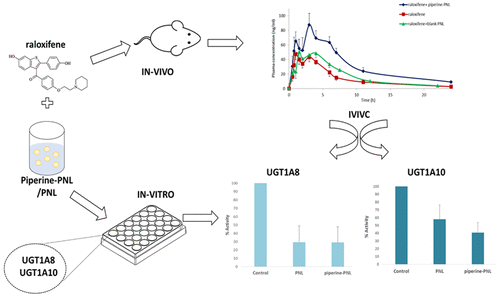
Phase II biotransformation reactions have been gaining more attention due to their acknowledged significance in drug bioavailability, drug development, and drug–drug interactions. However, the predominant role of phase I metabolism has always overshadowed phase II metabolism, resulting in insufficient data regarding its mechanisms. In this paper, we investigate the effect of an advanced lipid based formulation on the phase II metabolism process of glucuronidation, occuring in the enterocytes monolayer. The investigated formulation is a self-emulsifying drug delivery system, termed pro-nano lipospheres, which contains the natural absorption enhancer piperine. To evaluate the effect of this formulation on direct glucuronidation we chose the model molecule raloxifene. First, glucuronidation is the main clearance pathway of this compound without involvement of preceding mechanisms. Second, raloxifene’s extensive glucuronidation site is primarily at the intestine. Raloxifene’s oral bioavailability was determined in a series of pharmacokinetic experiments using the freely moving rat model. In order to test the effect of the formulation on the relevant UGT enzymes reported in the clinic, we used the in vitro method of UGT-Glo Assay. Coadministration of raloxifene and piperine pro-nano lipospheres to rats resulted in a 2-fold increase in the relative oral bioavailability of raloxifene. However, coadministration of raloxifene with blank pro-nano lipospheres had no effect on its oral bioavailability. In contrast to the difference found in vivo between the two vehicles, both formulations extended an inhibitory effect on UGT enzymes in vitro. Ultimately, these findings prove the ability of the formulation to diminish intestinal direct phase II metabolism which serves as an absorption obstacle for many of today’s marketed drugs. Pro-nano lipospheres is a formulation that serves as a platform for the simultaneous delivery of the absorption enhancer and a required drug. The discrepancy found between the in vivo and in vitro models demonstrates that the in vitro method may not be sensitive enough to distinguish the difference between the formulations.
中文翻译:

胡椒碱前纳米脂质体对直接肠道II期代谢的影响:雷洛昔芬范例具有增强的口服生物利用度
由于II期生物转化反应在药物生物利用度,药物开发和药物-药物相互作用中具有公认的重要性,因此受到越来越多的关注。但是,I期新陈代谢的主要作用始终掩盖了II期新陈代谢,导致有关其机理的数据不足。在本文中,我们研究了基于脂质的高级制剂对糖尿酸化作用的II期代谢过程的影响,该过程发生在肠单层细胞中。研究的制剂是一种自乳化的药物输送系统,称为天然乳脂球体,其中含有天然吸收促进剂胡椒碱。为了评估该制剂对直接葡萄糖醛酸化的作用,我们选择了模型分子雷洛昔芬。第一的,葡萄糖醛酸苷化是该化合物的主要清除途径,而没有涉及先前的机制。其次,雷洛昔芬的广泛葡萄糖醛酸化位点主要在肠道。使用自由移动的大鼠模型,在一系列药代动力学实验中确定了雷洛昔芬的口服生物利用度。为了测试该制剂对临床报告的相关UGT酶的影响,我们使用了UGT-Glo分析的体外方法。雷洛昔芬和胡椒碱前纳米脂质球共同给药于大鼠导致雷洛昔芬的相对口服生物利用度增加了2倍。但是,雷洛昔芬与空白的天然纳米脂质球并用对其口服生物利用度没有影响。与两种载体在体内发现的差异相反,两种制剂均在体外扩展了对UGT酶的抑制作用。最终,这些发现证明了该制剂减少肠道直接II期新陈代谢的能力,这成为当今许多市售药物的吸收障碍。前纳米脂球是一种制剂,可作为同时递送吸收促进剂和所需药物的平台。体内模型与体外模型之间的差异表明,体外方法可能不够灵敏,无法区分制剂之间的差异。
更新日期:2018-03-14
中文翻译:

胡椒碱前纳米脂质体对直接肠道II期代谢的影响:雷洛昔芬范例具有增强的口服生物利用度
由于II期生物转化反应在药物生物利用度,药物开发和药物-药物相互作用中具有公认的重要性,因此受到越来越多的关注。但是,I期新陈代谢的主要作用始终掩盖了II期新陈代谢,导致有关其机理的数据不足。在本文中,我们研究了基于脂质的高级制剂对糖尿酸化作用的II期代谢过程的影响,该过程发生在肠单层细胞中。研究的制剂是一种自乳化的药物输送系统,称为天然乳脂球体,其中含有天然吸收促进剂胡椒碱。为了评估该制剂对直接葡萄糖醛酸化的作用,我们选择了模型分子雷洛昔芬。第一的,葡萄糖醛酸苷化是该化合物的主要清除途径,而没有涉及先前的机制。其次,雷洛昔芬的广泛葡萄糖醛酸化位点主要在肠道。使用自由移动的大鼠模型,在一系列药代动力学实验中确定了雷洛昔芬的口服生物利用度。为了测试该制剂对临床报告的相关UGT酶的影响,我们使用了UGT-Glo分析的体外方法。雷洛昔芬和胡椒碱前纳米脂质球共同给药于大鼠导致雷洛昔芬的相对口服生物利用度增加了2倍。但是,雷洛昔芬与空白的天然纳米脂质球并用对其口服生物利用度没有影响。与两种载体在体内发现的差异相反,两种制剂均在体外扩展了对UGT酶的抑制作用。最终,这些发现证明了该制剂减少肠道直接II期新陈代谢的能力,这成为当今许多市售药物的吸收障碍。前纳米脂球是一种制剂,可作为同时递送吸收促进剂和所需药物的平台。体内模型与体外模型之间的差异表明,体外方法可能不够灵敏,无法区分制剂之间的差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号