当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed Synthesis of Imines and Esters from Benzyl Alcohols and Nitroarenes: Change in Catalyst Reactivity Depending on the Presence or Absence of the Phosphine Ligand
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.joc.8b00197 Taemoon Song 1 , Ji Eun Park 1 , Young Keun Chung 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.joc.8b00197 Taemoon Song 1 , Ji Eun Park 1 , Young Keun Chung 1
Affiliation
|
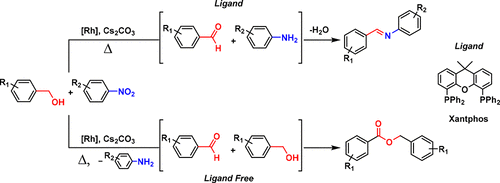
The [Rh(COD)Cl]2/xantphos/Cs2CO3 system efficiently catalyzes the reductive N-alkylation of aryl nitro compounds with alcohols by a borrowing-hydrogen strategy to afford the corresponding imine products in good to excellent yields. In the absence of xantphos, the [Rh(COD)Cl]2/Cs2CO3 catalytic system behaves as an effective catalyst for the dehydrogenative coupling of alcohols to esters, with nitrobenzene as a hydrogen acceptor. The reactivity of the rhodium catalytic system can be easily manipulated to selectively afford the imine or ester.
中文翻译:

铑催化的苄醇和硝基芳烃合成亚胺和酯:取决于是否存在膦配体,催化剂反应性的变化
[Rh(COD)Cl] 2 / xantphos / Cs 2 CO 3系统通过借位氢策略有效催化芳基硝基化合物与醇的还原性N-烷基化,从而以良好或优异的收率提供相应的亚胺产品。在不存在黄药的情况下,[Rh(COD)Cl] 2 / Cs 2 CO 3催化体系可作为醇与酯脱氢偶联的有效催化剂,硝基苯为氢受体。铑催化体系的反应性可以很容易地控制以选择性地提供亚胺或酯。
更新日期:2018-03-14
中文翻译:

铑催化的苄醇和硝基芳烃合成亚胺和酯:取决于是否存在膦配体,催化剂反应性的变化
[Rh(COD)Cl] 2 / xantphos / Cs 2 CO 3系统通过借位氢策略有效催化芳基硝基化合物与醇的还原性N-烷基化,从而以良好或优异的收率提供相应的亚胺产品。在不存在黄药的情况下,[Rh(COD)Cl] 2 / Cs 2 CO 3催化体系可作为醇与酯脱氢偶联的有效催化剂,硝基苯为氢受体。铑催化体系的反应性可以很容易地控制以选择性地提供亚胺或酯。











































 京公网安备 11010802027423号
京公网安备 11010802027423号