当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Observation of Radical Rebound in a Mononuclear Nonheme Iron Model Complex
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-14 , DOI: 10.1021/jacs.7b12707 Thomas M Pangia 1 , Casey G Davies 2 , Joshua R Prendergast 2, 3 , Jesse B Gordon 1 , Maxime A Siegler 1 , Guy N L Jameson 2, 3 , David P Goldberg 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-14 , DOI: 10.1021/jacs.7b12707 Thomas M Pangia 1 , Casey G Davies 2 , Joshua R Prendergast 2, 3 , Jesse B Gordon 1 , Maxime A Siegler 1 , Guy N L Jameson 2, 3 , David P Goldberg 1
Affiliation
|
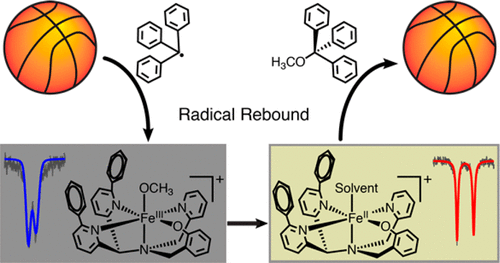
A nonheme iron(III) terminal methoxide complex, [FeIII(N3PyO2Ph)(OCH3)]ClO4, was synthesized. Reaction of this complex with the triphenylmethyl radical (Ph3C•) leads to formation of Ph3COCH3 and the one-electron-reduced iron(II) center, as seen by UV-vis, EPR, 1H NMR, and Mössbauer spectroscopy. These results indicate that homolytic Fe-O bond cleavage occurs together with C-O bond formation, providing a direct observation of the "radical rebound" process proposed for both biological and synthetic nonheme iron centers.
中文翻译:

单核非血红素铁模型复合物中自由基反弹的观察
合成了非血红素铁(III)末端甲醇复合物[FeIII(N3PyO2Ph)(OCH3)]ClO4。该配合物与三苯甲基自由基 (Ph3C•) 的反应导致形成 Ph3COCH3 和单电子还原铁 (II) 中心,如 UV-vis、EPR、1H NMR 和穆斯堡尔光谱所示。这些结果表明,均裂 Fe-O 键断裂与 CO 键形成同时发生,提供了对生物和合成非血红素铁中心提出的“自由基反弹”过程的直接观察。
更新日期:2018-03-14
中文翻译:

单核非血红素铁模型复合物中自由基反弹的观察
合成了非血红素铁(III)末端甲醇复合物[FeIII(N3PyO2Ph)(OCH3)]ClO4。该配合物与三苯甲基自由基 (Ph3C•) 的反应导致形成 Ph3COCH3 和单电子还原铁 (II) 中心,如 UV-vis、EPR、1H NMR 和穆斯堡尔光谱所示。这些结果表明,均裂 Fe-O 键断裂与 CO 键形成同时发生,提供了对生物和合成非血红素铁中心提出的“自由基反弹”过程的直接观察。











































 京公网安备 11010802027423号
京公网安备 11010802027423号