当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Withaferin A and Withanolide D Analogues with Dual Heat-Shock-Inducing and Cytotoxic Activities: Semisynthesis and Biological Evaluation
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00918 E. M. Kithsiri Wijeratne 1 , Maria C. F. Oliveira 1, 2 , Jair Mafezoli 1, 2 , Ya-Ming Xu 1 , Sandro Minguzzi 1, 3 , Pedro H. J. Batista 2 , Otília D. L. Pessoa 2 , Luke Whitesell 4 , A. A. Leslie Gunatilaka 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jnatprod.7b00918 E. M. Kithsiri Wijeratne 1 , Maria C. F. Oliveira 1, 2 , Jair Mafezoli 1, 2 , Ya-Ming Xu 1 , Sandro Minguzzi 1, 3 , Pedro H. J. Batista 2 , Otília D. L. Pessoa 2 , Luke Whitesell 4 , A. A. Leslie Gunatilaka 1
Affiliation
|
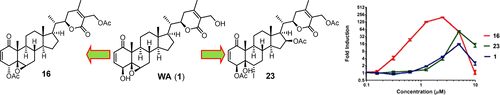
Withanolides constitute a valuable class of bioactive natural products because some members of the class are known to exhibit cytotoxic activity and also induce a cytoprotective heat-shock response. In order to understand the relationship between their structures and these dual bioactivities of the withanolide scaffold, we obtained 25 analogues of withaferin A (WA) and withanolide D (WD) including 17 new compounds by semisynthesis involving chemical and microbial transformations. Hitherto unknown 16β-hydroxy analogues of WA and WD were prepared by their reaction with triphenylphosphine/iodine, providing unexpected 5β-hydroxy-6α-iodo analogues (iodohydrins) followed by microbial biotransformation with Cunninghamella echinulata and base-catalyzed cyclization of the resulting 16β-hydroxy iodohydrins. Evaluation of these 25 withanolide analogues for their cytotoxicity and heat-shock-inducing activity (HSA) confirmed the known structure–activity relationships for WA-type withanolides and revealed that WD analogues were less active in both assays compared to their corresponding WA analogues. The 5β,6β-epoxide moiety of withanolides contributed to their cytotoxicity but not HSA. Introduction of a 16β-OAc group to 4,27-di-O-acetyl-WA enhanced cytotoxicity and decreased HSA, whereas introduction of the same group to 4-O-acetyl-WD decreased both activities.
中文翻译:

具有双重热休克诱导和细胞毒活性的Withaferin A和Withanolide D类似物:半合成和生物学评估
Withanolides构成一类有价值的生物活性天然产物,因为已知该类化合物的某些成员表现出细胞毒活性,并且还诱导细胞保护性热休克反应。为了了解其结构与withanolide支架的双重生物活性之间的关系,我们通过涉及化学和微生物转化的半合成获得了25种withaferin A(WA)和withanolide D(WD)的类似物,包括17种新化合物。通过与三苯基膦/碘反应制备了迄今未知的WA和WD16β-羟基类似物,提供了出乎意料的5β-羟基-6α-碘类似物(碘代醇),然后用棘枝棘孢菌进行了微生物生物转化。和碱催化环化所得的16β-羟基碘醇。对这25种withanolide类似物的细胞毒性和热激诱导活性(HSA)进行评估,证实了WA型withanolides具有已知的结构-活性关系,并揭示了在两种测定法中WD类似物与其相应的WA类似物相比活性较低。醇化物的5β,6β-环氧部分有助于其细胞毒性,但对HSA无贡献。一个16β-OAC基团的引入到4,27二ø -乙酰基WA增强的细胞毒性和降低的HSA,而导入同一组的至4- ö乙酰基WD降低两种活性。
更新日期:2018-03-14
中文翻译:

具有双重热休克诱导和细胞毒活性的Withaferin A和Withanolide D类似物:半合成和生物学评估
Withanolides构成一类有价值的生物活性天然产物,因为已知该类化合物的某些成员表现出细胞毒活性,并且还诱导细胞保护性热休克反应。为了了解其结构与withanolide支架的双重生物活性之间的关系,我们通过涉及化学和微生物转化的半合成获得了25种withaferin A(WA)和withanolide D(WD)的类似物,包括17种新化合物。通过与三苯基膦/碘反应制备了迄今未知的WA和WD16β-羟基类似物,提供了出乎意料的5β-羟基-6α-碘类似物(碘代醇),然后用棘枝棘孢菌进行了微生物生物转化。和碱催化环化所得的16β-羟基碘醇。对这25种withanolide类似物的细胞毒性和热激诱导活性(HSA)进行评估,证实了WA型withanolides具有已知的结构-活性关系,并揭示了在两种测定法中WD类似物与其相应的WA类似物相比活性较低。醇化物的5β,6β-环氧部分有助于其细胞毒性,但对HSA无贡献。一个16β-OAC基团的引入到4,27二ø -乙酰基WA增强的细胞毒性和降低的HSA,而导入同一组的至4- ö乙酰基WD降低两种活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号