Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2018-03-15 , DOI: 10.1016/j.bmcl.2018.03.035 Xiaolei Wang , Larissa Krasnova , Kevin Binchia Wu , Wei-Shen Wu , Ting-Jen Cheng , Chi-Huey Wong
|
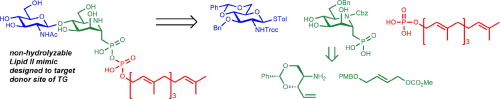
Described here is the asymmetric synthesis of iminosugar 2b, a Lipid II analog, designed to mimic the transition state of transglycosylation catalyzed by the bacterial transglycosylase. The high density of functional groups, together with a rich stereochemistry, represents an extraordinary challenge for chemical synthesis. The key 2,6-anti- stereochemistry of the iminosugar ring was established through an iridium-catalyzed asymmetric allylic amination. The developed synthetic route is suitable for the synthesis of focused libraries to enable the structure–activity relationship study and late-stage modification of iminosugar scaffold with variable lipid, peptide and sugar substituents. Compound 2b showed 70% inhibition of transglycosylase from Acinetobacter baumannii, providing a basis for further improvement.
中文翻译:

针对细菌转糖基酶的新型抗生素:合成脂质 II 类似物作为稳定的过渡态模拟抑制剂
这里描述的是亚氨基糖2b 的不对称合成,亚氨基糖 2b是一种脂质 II 类似物,旨在模拟细菌转糖基酶催化的转糖基化的过渡状态。高密度的官能团以及丰富的立体化学对化学合成来说是一个巨大的挑战。亚氨基糖环的关键2,6-反立体化学是通过铱催化的不对称烯丙胺化建立的。所开发的合成路线适用于合成重点库,以便能够进行构效关系研究以及具有可变脂质、肽和糖取代基的亚氨基糖支架的后期修饰。化合物2b对鲍曼不动杆菌转糖基酶有70%的抑制作用,为进一步改进提供了基础。









































 京公网安备 11010802027423号
京公网安备 11010802027423号