当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Throughput Isolation of Circulating Tumor Cells Using Cascaded Inertial Focusing Microfluidic Channel
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.analchem.7b04210 Aynur Abdulla 1 , Wenjia Liu 1 , Azarmidokht Gholamipour-Shirazi 1 , Jiahui Sun 1 , Xianting Ding 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.analchem.7b04210 Aynur Abdulla 1 , Wenjia Liu 1 , Azarmidokht Gholamipour-Shirazi 1 , Jiahui Sun 1 , Xianting Ding 1
Affiliation
|
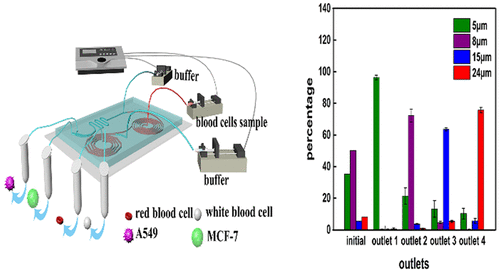
Circulating tumor cells (CTCs) are rare cells that detach from a primary or metastasis tumor and flow into the bloodstream. Intact and viable tumor cells are needed for genetic characterization of CTCs, new drug development, and other research. Although separation of CTCs using spiral channel with two outlets has been reported, few literature demonstrated simultaneous isolation of different types of CTCs from human blood using cascaded inertial focusing microfluidic channel. Herein, we introduce a cascaded microfluidic device consisting of two spiral channels and one zigzag channel designed with different fluid fields, including lift force, Dean drag force, and centrifugal force. Both red blood cells (RBCs)-lysed human blood spiked with CTCs and 1:50 diluted human whole blood spiked with CTCs were tested on the presented chip. This chip successfully separated RBCs, white blood cells (WBCs), and two different types of tumor cells (human lung cancer cells (A549) and human breast cancer cells (MCF-7)) simultaneously based on their physical properties. A total of 80.75% of A549 and 73.75% of MCF-7 were faithfully separated from human whole blood. Furthermore, CTCs gathered from outlets could propagate and remained intact. The cell viability of A549 and MCF-7 were 95% and 98%, respectively. The entire separating process for CTCs from blood cells could be finished within 20 min. The cascaded microfluidic device introduced in this study serves as a novel platform for simultaneous isolation of multiple types of CTCs from patient blood.
中文翻译:

级联惯性聚焦微流控通道高通量分离循环肿瘤细胞
循环肿瘤细胞(CTC)是稀有细胞,与原发性或转移性肿瘤分离并流入血流。CTC的遗传表征,新药开发和其他研究需要完整而可行的肿瘤细胞。尽管已经报道了使用带有两个出口的螺旋通道分离四氯化碳的方法,但是很少有文献证明使用级联惯性聚焦微流体通道同时从人血中分离出不同类型的四氯化碳。在此,我们介绍一种由两个螺旋通道和一个之字形通道组成的级联微流控设备,这些通道设计有不同的流体场,包括升力,迪安拖曳力和离心力。在提供的芯片上测试了两种掺有CTC的红细胞(RBC)溶解的人血和掺有CTC的1:50稀释的人全血。该芯片根据物理特性成功地成功分离了RBC,白细胞(WBC)和两种不同类型的肿瘤细胞(人肺癌细胞(A549)和人乳腺癌细胞(MCF-7))。从人类全血中忠实地分离出了80.75%的A549和73.75%的MCF-7。此外,从出口处收集到的四氯化碳可以传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了一种从患者血液中同时分离多种CTC的新型平台。以及两种不同类型的肿瘤细胞(人肺癌细胞(A549)和人乳腺癌细胞(MCF-7))同时根据其物理特性而定。从人类全血中忠实地分离出了80.75%的A549和73.75%的MCF-7。此外,从出口处收集到的四氯化碳可能会传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了从患者血液中同时分离多种CTC的新型平台。以及两种不同类型的肿瘤细胞(人肺癌细胞(A549)和人乳腺癌细胞(MCF-7))同时根据其物理特性而定。从人类全血中忠实地分离出了80.75%的A549和73.75%的MCF-7。此外,从出口处收集到的四氯化碳可能会传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了一种从患者血液中同时分离多种CTC的新型平台。从出口处收集到的四氯化碳可以传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了一种从患者血液中同时分离多种CTC的新型平台。从出口处收集到的四氯化碳可以传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了从患者血液中同时分离多种CTC的新型平台。
更新日期:2018-03-14
中文翻译:

级联惯性聚焦微流控通道高通量分离循环肿瘤细胞
循环肿瘤细胞(CTC)是稀有细胞,与原发性或转移性肿瘤分离并流入血流。CTC的遗传表征,新药开发和其他研究需要完整而可行的肿瘤细胞。尽管已经报道了使用带有两个出口的螺旋通道分离四氯化碳的方法,但是很少有文献证明使用级联惯性聚焦微流体通道同时从人血中分离出不同类型的四氯化碳。在此,我们介绍一种由两个螺旋通道和一个之字形通道组成的级联微流控设备,这些通道设计有不同的流体场,包括升力,迪安拖曳力和离心力。在提供的芯片上测试了两种掺有CTC的红细胞(RBC)溶解的人血和掺有CTC的1:50稀释的人全血。该芯片根据物理特性成功地成功分离了RBC,白细胞(WBC)和两种不同类型的肿瘤细胞(人肺癌细胞(A549)和人乳腺癌细胞(MCF-7))。从人类全血中忠实地分离出了80.75%的A549和73.75%的MCF-7。此外,从出口处收集到的四氯化碳可以传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了一种从患者血液中同时分离多种CTC的新型平台。以及两种不同类型的肿瘤细胞(人肺癌细胞(A549)和人乳腺癌细胞(MCF-7))同时根据其物理特性而定。从人类全血中忠实地分离出了80.75%的A549和73.75%的MCF-7。此外,从出口处收集到的四氯化碳可能会传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了从患者血液中同时分离多种CTC的新型平台。以及两种不同类型的肿瘤细胞(人肺癌细胞(A549)和人乳腺癌细胞(MCF-7))同时根据其物理特性而定。从人类全血中忠实地分离出了80.75%的A549和73.75%的MCF-7。此外,从出口处收集到的四氯化碳可能会传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了一种从患者血液中同时分离多种CTC的新型平台。从出口处收集到的四氯化碳可以传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了一种从患者血液中同时分离多种CTC的新型平台。从出口处收集到的四氯化碳可以传播并保持完整。A549和MCF-7的细胞活力分别为95%和98%。从血细胞中分离四氯化碳的整个过程可以在20分钟内完成。这项研究中介绍的级联微流控设备充当了从患者血液中同时分离多种CTC的新型平台。











































 京公网安备 11010802027423号
京公网安备 11010802027423号