当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fostering the Basic Instinct of Boron in Boron–Beryllium Interactions
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jpca.8b01551 M. Merced Montero-Campillo 1 , Ibon Alkorta 1 , José Elguero 1
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jpca.8b01551 M. Merced Montero-Campillo 1 , Ibon Alkorta 1 , José Elguero 1
Affiliation
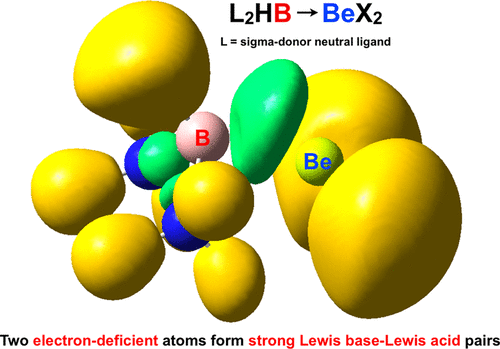
|
A set of complexes L2HB···BeX2 (L = CNH, CO, CS, N2, NH3, NCCH3, PH3, PF3, PMe3, OH2; X = H, F) containing a boron–beryllium bond is described at the M06-2X/6-311+G(3df,2pd)//M062-2X/6-31+G(d) level of theory. In this quite unusual bond, boron acts as a Lewis base and beryllium as a Lewis acid, reaching binding energies up to −283.3 kJ/mol ((H2O)2HB···BeF2). The stabilization of these complexes is possible thanks to the σ-donor role of the L ligands in the L2HB···BeX2 structures and the powerful acceptor nature of beryllium. According to the topology of the density, these B–Be interactions present positive laplacian values and negative energy densities, covering different degrees of electron sharing. ELF calculations allowed measuring the population in the interboundary B–Be region, which varies between 0.20 and 2.05 electrons upon switching from the weakest ((CS)2HB···BeH2) to the strongest complex ((H2O)2HB···BeF2). These B–Be interactions can be considered as beryllium bonds in most cases.
中文翻译:

在硼与铍相互作用中培养硼的基本本能
一组配合物L 2 HB···BeX 2(L = CNH,CO,CS,N 2,NH 3,NCCH 3,PH 3,PF 3,PMe 3,OH 2; X = H,F)硼-铍键在M06-2X / 6-311 + G(3df,2pd)// M062-2X / 6-31 + G(d)的理论水平上得到了描述。在这个非常不寻常的键中,硼充当路易斯碱,铍充当路易斯酸,达到高达-283.3 kJ / mol((H 2 O)2 HB···BeF 2)的结合能。由于L 2 HB···BeX 2中L配体的σ供体作用,这些配合物的稳定化成为可能。的结构和铍的强大受体性质。根据密度的拓扑结构,这些B–Be相互作用呈现正的拉普拉斯值和负的能量密度,涵盖了不同程度的电子共享。ELF计算允许测量边界B-Be区域中的人口,该区域在从最弱的((CS)2 HB···BeH 2)转换为最强的复合物((H 2 O)2 HB时,在0.20和2.05个电子之间变化···BeF 2)。在大多数情况下,这些B–Be相互作用可被视为铍键。
更新日期:2018-03-14
中文翻译:

在硼与铍相互作用中培养硼的基本本能
一组配合物L 2 HB···BeX 2(L = CNH,CO,CS,N 2,NH 3,NCCH 3,PH 3,PF 3,PMe 3,OH 2; X = H,F)硼-铍键在M06-2X / 6-311 + G(3df,2pd)// M062-2X / 6-31 + G(d)的理论水平上得到了描述。在这个非常不寻常的键中,硼充当路易斯碱,铍充当路易斯酸,达到高达-283.3 kJ / mol((H 2 O)2 HB···BeF 2)的结合能。由于L 2 HB···BeX 2中L配体的σ供体作用,这些配合物的稳定化成为可能。的结构和铍的强大受体性质。根据密度的拓扑结构,这些B–Be相互作用呈现正的拉普拉斯值和负的能量密度,涵盖了不同程度的电子共享。ELF计算允许测量边界B-Be区域中的人口,该区域在从最弱的((CS)2 HB···BeH 2)转换为最强的复合物((H 2 O)2 HB时,在0.20和2.05个电子之间变化···BeF 2)。在大多数情况下,这些B–Be相互作用可被视为铍键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号