当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Leader Peptide Binding During Catalysis by the Nisin Dehydratase NisB
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-14 , DOI: 10.1021/jacs.7b13506 Lindsay M. Repka 1 , Kenton J. Hetrick 1 , See Hyun Chee 1 , Wilfred A. van der Donk 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2018-03-14 , DOI: 10.1021/jacs.7b13506 Lindsay M. Repka 1 , Kenton J. Hetrick 1 , See Hyun Chee 1 , Wilfred A. van der Donk 1
Affiliation
|
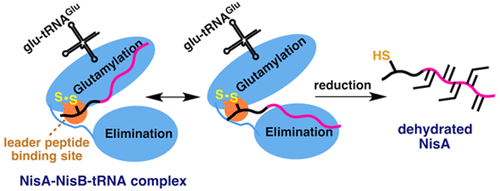
The dehydratase NisB performs stepwise tRNAGlu-dependent glutamylation of Ser/Thr residues and subsequent glutamate elimination to effect eight dehydrations in the biosynthesis of the antibacterial peptide nisin. Its substrate, NisA, bears a C-terminal core peptide that is modified and an N-terminal leader peptide (LP) that is not modified but that is required for efficient dehydration. To elucidate the mechanism of LP-NisB interactions during dehydration, we engineered a disulfide that covalently links the NisA LP to NisB. The enzyme fully dehydrated tethered NisA, confirming the functional LP binding site and supporting a mechanism where NisB uses a single LP binding site for glutamylation and elimination. We also show an order of NisA and tRNAGlu binding to NisB that enables dehydration.
中文翻译:

Nisin 脱水酶 NisB 催化过程中前导肽结合的表征
脱水酶 NisB 执行 Ser/Thr 残基的逐步 tRNAGlu 依赖性谷氨酰化和随后的谷氨酸消除,以在抗菌肽乳酸链球菌肽的生物合成中实现八次脱水。它的底物 NisA 带有一个经过修饰的 C 端核心肽和一个未经修饰但有效脱水所需的 N 端前导肽 (LP)。为了阐明脱水过程中 LP-NisB 相互作用的机制,我们设计了一种二硫化物,将 NisA LP 与 NisB 共价连接。该酶完全脱水束缚 NisA,确认功能性 LP 结合位点并支持 NisB 使用单个 LP 结合位点进行谷氨酰化和消除的机制。我们还展示了 NisA 和 tRNAGlu 与 NisB 结合的顺序,从而使脱水成为可能。
更新日期:2018-03-14
中文翻译:

Nisin 脱水酶 NisB 催化过程中前导肽结合的表征
脱水酶 NisB 执行 Ser/Thr 残基的逐步 tRNAGlu 依赖性谷氨酰化和随后的谷氨酸消除,以在抗菌肽乳酸链球菌肽的生物合成中实现八次脱水。它的底物 NisA 带有一个经过修饰的 C 端核心肽和一个未经修饰但有效脱水所需的 N 端前导肽 (LP)。为了阐明脱水过程中 LP-NisB 相互作用的机制,我们设计了一种二硫化物,将 NisA LP 与 NisB 共价连接。该酶完全脱水束缚 NisA,确认功能性 LP 结合位点并支持 NisB 使用单个 LP 结合位点进行谷氨酰化和消除的机制。我们还展示了 NisA 和 tRNAGlu 与 NisB 结合的顺序,从而使脱水成为可能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号