Innovative Food Science & Emerging Technologies ( IF 6.6 ) Pub Date : 2018-03-14 , DOI: 10.1016/j.ifset.2018.03.015 Bonastre Oliete , François Potin , Eliane Cases , Rémi Saurel
|
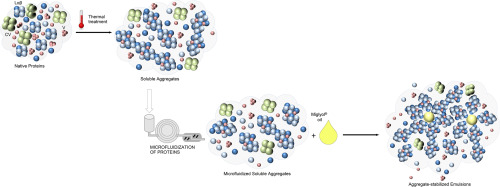
The effects of thermal aggregation and microfluidization on pea (Pisum sativum L.) globulin characteristics and emulsifying properties were investigated. The thermal treatment of native pea globulins (NPs) induced the formation of aggregates (<150 nm) that were partially stabilized by disulphide bonds and characterized by higher surface hydrophobicity and lower surface charge vs NPs. These modified characteristics facilitated the development of O/W interfaces and smaller droplets by aggregates. Nevertheless, coalescence and flocculation were favoured in aggregate-based vs NP-based emulsions. The microfluidization process (70, 130 MPa) led to structure rearrangements within globulin aggregates, resulting in decreased protein particle size and hydrophobicity. The positive action of the new characteristics of microfluidized aggregates on emulsion stability, by reducing the flocculation and creaming phenomena, was more pronounced at the highest microfluidization pressure. Because of these enhanced inter-droplet interactions, the gel-like structure of the aggregate-stabilized emulsion was supposed to play a key role in this stability.
Industrial relevance
Microfluidization or dynamic high pressure is a novel technology that could improve techno-functional properties of pea proteins after thermal aggregation. Microfluidization process at 70 MPA or 130 MPa broke large aggregates previously induced by the protein aggregation, and lead to new protein conformations. The new characteristics of microfluidized aggregates partly hinder the negative effect of thermal aggregation on the emulsion stability of aggregated proteins, especially when high microfluidization pressures were used (130 MPa). These findings will be of crucial importance for the development of pea protein-based oily formulations.
中文翻译:

动态高压流化对豌豆球蛋白可溶性聚集体乳化性能的调节
热聚集和微流化对豌豆(Pisum sativum L.)的影响。)研究了球蛋白的特性和乳化性能。天然豌豆球蛋白(NPs)的热处理诱导形成聚集体(<150 nm),该聚集体通过二硫键部分稳定,并且与NPs相比,具有较高的表面疏水性和较低的表面电荷。这些改进的特性促进了O / W界面的发展以及聚集体产生的较小液滴。然而,聚集体和絮凝在基于聚集体的乳液与基于NP的乳液中是有利的。微流化过程(70、130 MPa)导致球蛋白聚集体内的结构重排,从而导致蛋白质粒径和疏水性降低。通过减少絮凝和乳化现象,微流化聚集体新特性对乳液稳定性的积极作用,在最高微流化压力下更明显。由于这些增强的液滴间相互作用,聚集体稳定的乳液的凝胶状结构被认为在这种稳定性中起关键作用。
行业相关性
微流化或动态高压是一种新技术,可以提高热聚集后豌豆蛋白的技术功能特性。70 MPA或130 MPa的微流化过程破坏了先前由蛋白质聚集诱导的大聚集体,并导致了新的蛋白质构象。微流化聚集体的新特性部分地阻碍了热聚集对聚集蛋白的乳液稳定性的负面影响,尤其是在使用高微流化压力(130 MPa)的情况下。这些发现对于基于豌豆蛋白的油性制剂的开发至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号