当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity Relationship Study on Odoamide: Insights into the Bioactivities of Aurilide-Family Hybrid Peptide–Polyketides
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00028 Masato Kaneda 1 , Shinsaku Kawaguchi 1 , Nobutaka Fujii 1 , Hiroaki Ohno 1 , Shinya Oishi 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acsmedchemlett.8b00028 Masato Kaneda 1 , Shinsaku Kawaguchi 1 , Nobutaka Fujii 1 , Hiroaki Ohno 1 , Shinya Oishi 1
Affiliation
|
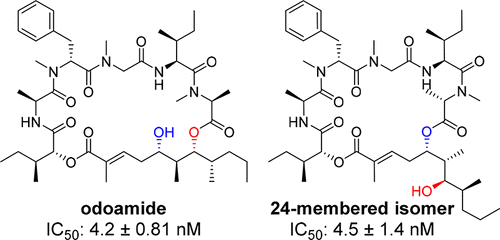
Odoamide is a cytotoxic peptide–polyketide hybrid molecule isolated from the Okinawan marine cyanobacterium Okeania sp. For an efficient structure–activity relationship study of the peptide part of odoamide, a facile synthetic protocol was established using a solid-phase peptide synthesis. Among a series of peptides, the d-MeAla6 isomer exhibited a more potent cytotoxicity than natural odoamide. It was also demonstrated that the 26-membered macrocyclic natural odoamide and the 24-membered isomer with comparable cytotoxicities were slowly interconvertible, and both isomers contributed to the potent cytotoxicity of odoamide. Examination of the physicochemical properties revealed that the in vitro cytotoxicity was affected by the serum protein binding of odoamide derivatives, while the differences in the macrocyclic structures had no significant effect on the membrane permeability.
中文翻译:

Odoamide的结构-活性关系研究:对Aurilide-家庭杂合肽-Polyketides的生物活性的见解。
Odoamide是从冲绳海洋蓝藻Okeania sp。中分离出来的一种细胞毒性肽-聚酮化合物杂合分子。为了有效地研究odoamide肽部分的结构-活性关系,使用固相肽合成方法建立了一种简便的合成方案。在一系列肽中,d-MeAla6异构体比天然的odamide具有更强的细胞毒性。还证明了具有可比的细胞毒性的26元大环天然邻酰胺和24元异构体可缓慢互变,并且这两种异构体均对odoamide具有强的细胞毒性。理化性质的检查表明,体外细胞毒性受odoamide衍生物的血清蛋白结合的影响,而大环结构的差异对膜的通透性没有显着影响。
更新日期:2018-03-05
中文翻译:

Odoamide的结构-活性关系研究:对Aurilide-家庭杂合肽-Polyketides的生物活性的见解。
Odoamide是从冲绳海洋蓝藻Okeania sp。中分离出来的一种细胞毒性肽-聚酮化合物杂合分子。为了有效地研究odoamide肽部分的结构-活性关系,使用固相肽合成方法建立了一种简便的合成方案。在一系列肽中,d-MeAla6异构体比天然的odamide具有更强的细胞毒性。还证明了具有可比的细胞毒性的26元大环天然邻酰胺和24元异构体可缓慢互变,并且这两种异构体均对odoamide具有强的细胞毒性。理化性质的检查表明,体外细胞毒性受odoamide衍生物的血清蛋白结合的影响,而大环结构的差异对膜的通透性没有显着影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号